|
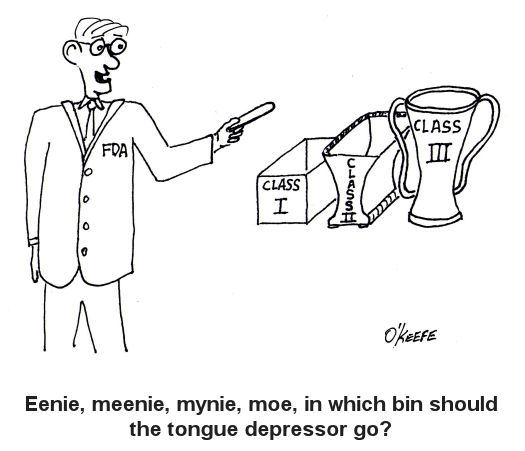
Hardly a week goes by that the FDA, that is the Food and Drug Administration, is not in the news. From the recall of drugs found to be harmful after the fact, to investigations of medical device suppliers and inspections of salmonella contamination at meat processing plants, the FDA is responsible for overseeing and regulating a wide range of products and processes. It’s stated purpose being: The FDA is responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit radiation. http://www.fda.gov/AboutFDA/WhatWeDo/default.htm From its humble beginnings at the beginning of the last century as the Pure Food and Drug Act, it has grown to regulate more than $1 trillion worth of consumer goods, about 25% of that said to be attributable to consumer goods expenditures in the United States. In 1976, the FDA began classifying medical devices, using a three-tiered system to distinguish them according to level of risk to patients. Class I devices present the lowest level of risk and requires the least regulatory control, while Classes II and III represent higher levels of risk. Just to give you some perspective, Class I medical devices include things like tongue depressors, bedpans, arm slings, and hand-held surgical instruments. In the Class II category are things like surgical drapes, blood pressure cuffs, catheters, wheelchairs, heating pads, and x-ray film processing machines. And I’m sure you’ve guessed by now that Class III devices are for the heavy-hitters, including things like defibrillators, heart valves, and implanted cerebral stimulators. So why does the FDA classify medical devices? Practicality is one key reason. It would be highly impractical, if not downright impossible, for the FDA to subject manufacturers of low risk Class I devices, like tongue depressors, to the same scrutiny as manufacturers of Class III devices, such as heart valves, etc. Along with miles of red tape would come a huge financial burden that would effectively raise the price of your common tongue depressor through the roof and, undoubtedly, force the manufacturer out of business. Hand in hand with medical device classifications are regulatory controls imposed by the FDA. We’ll see how they fit into the picture next time. _____________________________________________ |
Engineering Expert Witness Blog
Published by Philip J. O'Keefe, PE, MLE