| I was out in the garage today spray painting, a job I would have preferred to have done outdoors, but alas, it was raining. It wasn’t a big job, and I probably didn’t spend more than about an hour doing it, but by the time I was done I was all too aware of how noxious the chemical fumes were that were put out by my aerosol spray can. I had thought that with all the garage doors open and a good cross breeze going through I’d be spared the unpleasant smell. Now imagine this all on a much larger scale, say an industrial setting, where massive spray painters are used all day long.
We’ve been talking for awhile now about filtration, from fabric filters to cyclones, and how they are most effective when integrated into a local exhaust ventilation system. These filtration devices are great for the removal of airborne particles like dust, but they don’t do a good job removing chemical vapors like paint fumes, much in the same way as a dust mask wouldn’t have made my spray painting job any less smelly. This week we’ll focus on filtration capable of addressing the special challenges presented by chemical vapors in the air. Chemical vapor contaminants can be separated from good air trapped in a local exhaust ventilation system by way of an air cleaner in a process known as absorption. In this instance, just like with our smelly goldfish tank, the media can consist of activated carbon, a carbon created by intense heating of substances like bituminous coal, wood, or coconut shell. The heat removes everything except carbon and creates myriads of tiny pores throughout. These pores give activated carbon tremendous surface area, meaning lots of nooks and crannies for chemical molecules to get lodged in. And when I say “lots” of nooks and crannies, I mean it. One pound of granular activated carbon has enough pores to give it a surface area of 125 acres! As the air-vapor mixture passes over the huge surface area, chemical vapors are absorbed by combining chemically with the carbon. Jamb packing surface area into a small space, as activated carbon does, creates a media capable of absorbing vast amounts of chemical molecules for a long time. As effective as this system is, the carbon pores will eventually become saturated with contaminants, and when it does, it is easily addressed. Simply replace the media with fresh carbon. Another means of removing harmful vapors from the air is through the use of an air cleaner employing temperature as its means of filtration. I’ll bet you’re asking how that works, and here’s an example you can relate to. It’s a hot, humid day, and the only thing standing between you and total discomfort is a glass of ice water. As you eagerly lift the glass to your lips, you notice the glass is wet on the outside, so wet that it’s actually dripping. In the stupor caused by your heat exhaustion you may for a moment think that the glass is actually leaking, but you soon realize that the water has accumulated on the outside of the glass because the hot, humid air that is making you so uncomfortable has also come into contact with the cold surface of the glass. When the water vapor in the atmosphere hits the cool of the glass filled with ice, it condenses into droplets. This condensation process stops when the glass temperature equalizes to that of the temperature in the surrounding air. Air cleaners can make use of the same phenomenon to filter contaminants. In their case the contaminated air mixture is cooled to the point where the humidity and chemical vapors present condense together to form a liquid, and the liquid is then drained out for proper disposal. That’s it for our look at filters and air cleaners. To sum things up, remember that there are a variety of factors that have to be considered when selecting filters and air cleaning devices. These include the volume of air flowing through the system, the concentration of contaminants in the air, the chemical and physical properties of the contaminants, the hazards associated with the contaminants, and the emissions standards established by federal, state, and local environmental regulations. Next time we’ll explore the workhorse of a local exhaust ventilation system, its fan. _____________________________________________ |
Posts Tagged ‘fumes’
Industrial Ventilation – Local Exhaust Ventilation Filters and Air Cleaners III
Sunday, May 15th, 2011Industrial Ventilation – Local Exhaust Ventilation Ductwork
Monday, April 25th, 2011| Ever venture into your basement and stare in amazement at the labyrinth of ductwork stemming off from the furnace? Believe it or not, there’s a science behind that spaghetti bowl configuration. Ductwork can either be flexible or rigid, square or round in shape. Its job in a local exhaust ventilation system is to carry airborne contaminants from the originating source, the carefully positioned hood in the workplace, to the exhaust stack where it is vented outside the building. This job isn’t an easy one. Fluids, like air thick with toxins and toxic gases, don’t want to flow very well through ductwork unless you make their path as unimpeded as possible.
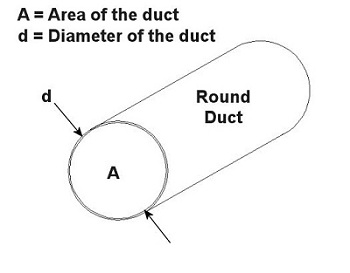
You can think of the air and contaminants flowing through ductwork as if it were like a car moving down a highway. Expressways don’t have sharp 90 degree turns or abrupt changes in width. These would cause a slow down in traffic, unless of course an accident is in the way. Expressways also tend to be rather large thoroughfares. The science behind ductwork follows the same basic principles to work effectively. It will minimize or eliminate sharp turns and it will avoid any abrupt changes in diameter. It will also be as wide in diameter as the environment will accommodate in order to move air volume most effectively. A local exhaust ventilation system’s performance can be greatly hampered and workers exposed to hazards if ductwork leaks. And if the leaks are upstream of the fan and large enough, they can reduce the ability of the local exhaust ventilation system to draw the airborne contaminants into the hood. Air starts getting drawn in through the leaks rather than through the hood. If the leaks are downstream of the fan, the airborne contaminants can re-enter the work area through the leaks rather than going outside through the exhaust stack. Ducts come in an endless variety of diameters, the diameter being part of a simple mathematical equation relating to flow velocity. In the simplest of terms, the flow of air through ductwork is governed by the following equation: Q = V × A where Q is the flow rate of air through the duct in cubic feet per minute (CFM), V is velocity of the air flow in feet per minute, and A is the cross sectional area of the duct in square feet. As an example, suppose you want to design a local exhaust ventilation system with ducts no greater than 5 inches in diameter because of space and clearance limitations. You want to use round ducts for the system because they handle air more efficiently and have no sharp corners where dust can collect. If an industrial hygienist determines that the air is required to flow at a minimum of 800 feet per minute through the duct, what is the airflow rate through the duct? Well, since we are dealing with a round duct, its cross sectional area is that of a circle: A = (π × d2) ÷ 4 where d is the diameter of the inside of the duct as shown in Figure 1 below. Figure 1 – Cross Section of a Round Duct So to use this equation for area, to solve for Q, then we must first convert the duct diameter from inches to feet, which makes our equation look like this: 5 inches ÷ 12 inches per foot = 0.416 feet This gives us a duct cross sectional area of: A = (π × (0.416 feet) 2) ÷ 4 = 0.136 square feet And the air will flow through the duct at this rate: Q = V × A = 800 ft./minute × 0.136 ft.2 = 108.8 CFM This air flow rate is good to know, because it will help the designer to select an appropriate fan for the local exhaust ventilation system. This is because fans are listed in manufacturers’ catalogs according to how many CFM they can handle. Next time, we’ll learn more about the rest of the local exhaust ventilation system, namely, the filter, fan, and exhaust stack. _____________________________________________ |
Industrial Ventilation – Dilution Ventilation
Sunday, April 3rd, 2011| If you’re a fan of the new hit HBO series, Boardwalk Empire, then you know a lot about the effects of Prohibition. But did you know that Prohibition is responsible for the creation of mixed drinks? Until then, people drank their liquor straight. Then along came Prohibition, mob rule, and the desire to keep the booze, including some with questionable origins, flowing. This booze didn’t taste so good, and the addition of a sugary beverage to it, that is, diluting it with soda or juice, made it a lot easier to go down. By the time Prohibition was repealed in 1933, the mixed drink had taken a firm foothold in American society.
Most adults are aware of the fact that liquor, in excess, is toxic to the body. Too much of it, and the liver, which acts as a filtration device, itself becomes toxic. When that happens, poor health will follow. The same principle applies to air within a building. If it becomes thick with toxic fumes or potentially flammable vapors, indoor air quality will suffer. But if you infuse fresh air into the environment, the toxic load is diluted, making the environment habitable and safe. This addition of fresh air is called, appropriately enough, dilution ventilation. Now, the easiest way to create a dilution ventilation system is to open a window. Trouble is there often isn’t enough natural airflow to do much good. But if you step up the effort by introducing a mechanical ventilation system, complete with blowers and ductwork, the need to crack open a window becomes obsolete. By exchanging bad air for good and introducing a continual flow of fresh air, toxicity is diluted and its effects are minimized, much like the bathtub gin of Prohibition was improved by the addition of soda. The chance of fire or explosion is reduced as well. There are however limits to what dilution ventilation can accomplish. If contaminants are highly toxic or extremely flammable, then this type of ventilation system is not going to do much good. This is a situation where extremely high air flows would be required, and this is often impractical both from a cost and comfort standpoint. Imagine having to work inside a wind tunnel? In situations like these a local exhaust ventilation system is better suited to do the job, and we’ll see how those work next week. _____________________________________________ |