Archive for the ‘Professional Malpractice’ Category
Sunday, May 26th, 2013
|
We’ve been discussing hurtles which must be jumped in order for an inventor’s creation to be considered for a patent. Federal statutes, namely 35 USC § 101, define the bases of patentability, including providing definitions on key terms, such as what constitutes a machine, an article of manufacture, and a composition of matter. Today we’ll wrap up our discussion on determining patent eligibility when we explore the final hurtle by defining process.
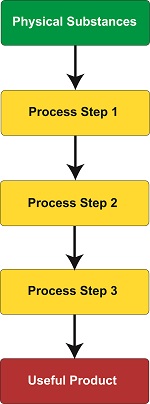
To get an understanding of what is meant by process, we must look to the lawsuit of Gottschalk v. Benson, a case involving patentability of a mathematical algorithm within a computer program. In this case the US Supreme Court held that a process is a series of steps or operations that transform substances or came about by way of a newly invented machine.
Based on the Court’s definition, a process can be many things, from a production line that transforms corn into corn chips within a food manufacturing plant to a mathematical algorithm running within software on the platform of a newly devised type of computer. However, the term usually pertains to a series of operations or steps, most frequently manufacturing in nature, where physical substances are transformed into useful products, that is, they possess the quality of utility, as discussed earlier in this blog series. A “physical substance” is anything of a physical nature existing on our planet.
Before I end this series I’d like to mention that under 35 USC § 101 an invention can be eligible for a patent if it makes a useful and beneficial improvement to an existing machine, article of manufacture, composition of matter, or process. That is to say, something may have already been patented which performs a specific function, but if that is improved upon in any significant way, it may receive a new patent.
For example, suppose an improved process for manufacturing food products was developed by adding additional steps to an existing patented process. If this improvement results in benefits such as lowered production costs, increased production rate, or reduced health risks to consumers, then this improved process may be eligible for a patent under 35 USC § 101.
Next time we’ll begin an exploration of the growing presence of 3D animations within the courtroom, specifically how they bring static 2D patent drawings to life.
___________________________________________
|
Tags: 35 USC § 101, 35 USC Section 101, 3D animations, article of manufacture, composition of matter, engineering expert witness, food manufacturing, food manufacturing plant, food processing, forensic engineer, health risks to consumers, lawsuit, machine, patent eligibility, patent infringement, patented process, process, process improvement, production costs, production rate, Title 35, useful article
Posted in Courtroom Visual Aids, Engineering and Science, Expert Witness, Innovation and Intellectual Property, Product Liability, Professional Malpractice | Comments Off on Determining Patent Eligibility – Part 7, Process
Sunday, May 12th, 2013
|
In high school chemistry class we watched a movie in which nothing less than a magical transformation took place. A scientist mixed two parts hydrogen gas and one part oxygen gas in a clear, sealed container, then sent an electrical charge into it. It created a spark, which provided the energy to force the two gases to join together in an explosive chemical union. The result was that they became a composition of matter which we recognize to be water.

Composition of matter is a term within the federal statute that determines patent eligibility, that of 35 USC § 101. It, along with the other terms we’ve been discussing, such as machine, and article of manufacture, is yet another consideration which must be addressed on the road to patentability.
To get a handle on the meaning of composition of matter, we have to go back to the Supreme Court’s ruling in Diamond v. Chakrabarty, a landmark case introduced in last week’s blog. Here the court defined composition of matter as, “compositions of two or more substances and all composite articles, whether they be the results of chemical union, or of mechanical mixture, or whether they be gases, fluids, powders or solids.” The Court’s definition of composition of matter covers chemical compounds and composites.
The Merriam-Webster Dictionary, defines a composite as something “made up of distinct parts.” Composite articles include most of the man-made products modern society is so familiar with and can’t seem to live without. Examples include plywood, concrete, and fiberglass. They’re typically made up of a myriad of components, some of which are raw materials, some man-made chemical compounds.
Chemical compounds are commonly made by uniting two or more chemical elements, the basic building blocks of matter that you might be familiar with from the Periodic Table always on display in a high school chemistry classroom. When a chemical union takes place the elements are forced, by way of mixing and heating, to bind together at the atomic level. If you’re not quite sure what “atomic” means, visit this site for a brief refresher: Atom Definition
Chemical compounds include man-made things like fuels, plastics, fertilizers, food preservatives, pesticides, and cleaning solutions. They’re all things that require human intervention to produce.
You may not realize it, but metal alloys are also essentially chemical compounds. These alloys are formed when two or more metals, or a metal and nonmetal, are fused together. Steel, for example, is an alloy composed of multiple elements, including iron, nickel, and carbon, which mix together during heating and become molten. During cooling the elements firmly unite and form atomic bonds to produce a new solid, one not available directly from nature.
Next time we’ll wrap up our discussion on 35 USC § 101by discussing the meaning of process with regard to patent eligibility.
___________________________________________
|
Tags: 35 USC § 101, 35 USC Section 101, article of manufacture, chemical compound, composite, Diamond v. Chakrabarty, engineering expert witness, forensic engineer, machine, metal alloy, patent eligibility, patent infringement, process
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Product Liability, Professional Malpractice | Comments Off on Determining Patent Eligibility – Part 6, Composition of Matter
Monday, May 6th, 2013
|
Imagine having freshly baked pastries available to you all day long, every day, while at work. I’m not talking about someone bringing in a box of donuts to share, I’m talking about baked goods on a massive scale. This is what I experienced in one of my design engineering positions within the food industry. These baked goods constituted the articles of manufacture of the food plant, and they presented a constant temptation to me.

Just what constitutes an article of manufacture is another aspect of the second hurtle which must be passed to determine patent eligibility. It is addressed under federal statutes governing the same, 35 USC § 101, and is contained within the same area as the discussion of what constitutes a machine, a subject we took up previously in this series.
Why bother defining articles of manufacture? Well, while hearing the patent case of Diamond v. Chakrabarty regarding genetically engineered bacterium capable of eating crude oil, the US Supreme Court saw fit to define the term so as to resolve a conflict between the inventor and the patent office as to whether a living organism could be patented.
The net result was the Court declared that in order to be deemed a patentable article of manufacture the object must be produced from either raw or man-made materials by either hand labor or machinery and must take on “new forms, qualities, properties, or combinations” that would not naturally occur without human intervention. In other words, a creation process must take place and something which did not previously exist must be caused to exist.
The court’s definition of articles of manufacture encompasses an incredible array of products, much too vast to enumerate here. Suffice it to say that the defining characteristic is that if it should consist of two or more parts, there is no interaction between the parts, otherwise it could be categorized as a machine. In other words, the relationship between their parts is static, unmoving. An example would be a hammer. It’s made up of two parts, a steel head and wooden handle. These parts are firmly attached to one another, so they act as one.
Next time we’ll continue our discussion on the second hurtle presented by 35 USC § 101, where we’ll discuss what is meant by composition of matter.
___________________________________________
|
Tags: 35 USC § 101, 35 USC Section 101, articles of manufacture, baked goods, design engineering, engineering expert witness, food industry, food manufacturing, forensic engineer, machine, manufactured articles, parts, patent, patent eligibility, patent infringement, process
Posted in Engineering and Science, Expert Witness, Innovation and Intellectual Property, Product Liability, Professional Malpractice | Comments Off on Determining Patent Eligibility – Part 5, Manufactured Articles
Sunday, April 28th, 2013
|
During 6th grade science we had a chapter on Simple Machines, and my textbook listed a common lever as an example, the sort that can be used to make work easier. Its illustration showed a stick perched atop a triangular shaped stone, appearing very much like a teeter-totter in the playground. A man was pushing down on one end of the stick to move a large boulder with the other end. Staring at it I thought to myself, “That doesn’t look like a machine to me. Where are its gears?” That day I learned about more than just levers, I learned to expect the unexpected when it comes to machines.
Last time we learned that under patent law the machine referred to in federal statute 35 USC § 101 includes any physical device consisting of two or more parts which dynamically interact with each other. We looked at how a purely mechanical machine, such as a diesel engine, has moving parts that are mechanically linked to dynamically interact when the engine runs. Now, lets move on to less obvious examples of what constitutes a machine.
Would you expect a modern electronic memory stick to be a machine? Probably not. But, under patent law it is. It’s an electronic device, and as such it’s made up of multiple parts, including integrated circuit chips, resistors, diodes, and capacitors, all of which are soldered to a printed circuit board where they interact with one another. They do so electrically, through changing current flow, rather than through physical movement of parts as in our diesel engine.
A transformer is an example of another type of machine. An electrical machine. Its fixed parts, including wire coils and steel cores, interact dynamically both electrically and magnetically in order to change voltage and current flow.
Electromechanical, the most complex of all machine types, includes the kitchen appliances in your home. They consist of both fixed and moving parts, along with all the dynamic interactions of mechanical, electronic, and electrical machines.
Next time we’ll continue our discussion on the second hurtle presented by 35 USC § 101, where we’ll discuss what is meant by article of manufacture.
___________________________________________
|
Tags: 35 USC § 101, 35 USC Section 101, capacitor, current flow, diode, electrically, electromechanical device, electronic, electronic device, engine, engineering expert witness, gears, integrated circuit chip, kitchen appliances, machine, machine design, magnetically, moving parts, patent eligibility, patent law, printed circuit board, resistor, simple machine, steel cores, Title 35 United States Code, transformer, utility patent, voltage, wire coils
Posted in Engineering and Science, Expert Witness, Innovation and Intellectual Property, Product Liability, Professional Malpractice | Comments Off on Determining Patent Eligibility – Part 4, Machines of a Different Kind
Sunday, April 21st, 2013
|
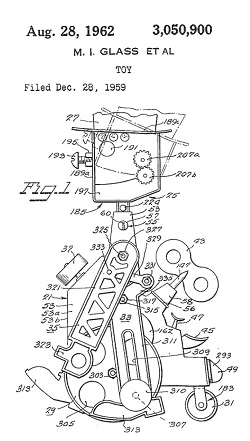
One of my favorite toys as a kid was Mr. Machine. He was a windup mechanical man that swung his arms when he walked while repeatedly squawking a strange YAK! sound. His body was transparent, so all the gears and levers inside were visible, and he even came with his own repair wrench. Alas, his wrench was of little use when Mr. Machine took a tragic fall down the basement stairs.
Mr. Machine was aptly named. There’s no question but that he was a machine, because his inventor received a US patent, No. 3,050,900. In order to accomplish this he had to have met guidelines set out in federal statutes, specifically those contained in 35 USC § 101. He had to prove that Mr. Machine was a bona fide machine.
If you’ll recall from last week’s discussion, in order to secure a patent, inventions must prove to be original technology that is classifiable as a machine, an article of manufacture, a composition of matter, or a process, or an improvement upon same. Last week our focus was on utility, the first hurdle that an invention must jump for it to be patent eligible. Let’s continue our discussion on patentability by examining the second hurtle.
When you consider the word machine, you might imagine something containing mechanical parts, like my childhood mechanical friend. But in the world of patents that’s not necessarily the case. There, a machine can be mechanical, electrical, electronic, or electromechanical in nature. In other words, a machine can include anything from a cell phone to a rocket.
To be precise, under patent law the definition of machine includes any physical device consisting of two or more parts which dynamically interact with each other. For example, a purely mechanical machine, such as a diesel engine, has many moving parts. Those parts, the pistons, connecting rods, etc., are mechanically linked to dynamically interact, or move together, when the engine runs.
Next week we’ll consider less obvious examples of what constitutes a machine under patent law.
___________________________________________
|
Tags: 35 USC § 101, 35 USC Section 101, article of manufacture, composition of matter, connecting rods, diesel engine, electrical engineer, engineerng expert witness, federal statutes, gears, improvement, levers, machine, mechanical engineer, mechanical machine, mechanically linked, moving parts, patent infringement, pistons, process, Title 35 United States Code, utility, utility patent, wrench
Posted in Engineering and Science, Expert Witness, Innovation and Intellectual Property, Product Liability, Professional Malpractice | Comments Off on Determining Patent Eligibility – Part 3, What Constitutes a Machine?
Sunday, March 24th, 2013
|
Who hasn’t finished a project, only to discover that you’d done something wrong and the whole thing would need to be redone? Perhaps you hadn’t checked your work along the way, confident that all would be well in the end. Imagine the costs involved if this scenario were to take place on a commercial production line. The Systems Engineering Approach to things helps ensure this doesn’t happen.
Last time we wrapped up our discussion on the Production stage of the systems engineering approach to medical device design, and today we’ll cover the final stage, Utilization.
The Utilization stage marks the point at which the medical device has been sold and is in actual use in the marketplace. Despite the fact that the product has at this point undergone many reviews and revisions and a great investment has been made into deciding whether or not to put it into production, changes can still take place in its design. Markets aren’t static, and products may be made to change due to stakeholders’, that is, those with a vested interest, changing requirements, whether those are aimed at further cost reduction, or perhaps to implement innovations to make the product more appealing to end users.
Other reasons for change may be initiated by the sales and marketing departments. They keep their fingers on the pulse of consumer trends, and they may want the design modified according to market research and feedback they receive from dealers, service technicians, and end users.
For example, the sales staff may have been apprised by end users that the keypad to their electronic muscle stimulating device needs modification. Patients have voiced they would prefer to here a clicking sound when depressing the buttons, in order to receive some auditory feedback. In addition, distributors of the device reported that although the electronic stimulators were functioning as intended, end users didn’t like the feel of the buttons. The lack of tactile feedback often led to confusion because they weren’t sure whether they had depressed the button or not.
Another interesting discovery concerning lack of feedback was that product service technicians were reporting premature wearing out of the keypads. Absent the satisfying click sound, users were inclined to push on the pads too strenuously, which drove up warranty service costs. The medical device manufacturer’s stakeholders are always concerned with costs, and increased service costs definitely raise the red flag.
Considerations like these typically arise after a medical device enters the Utilization stage. Fortunately, the objective of the systems engineering approach is to ensure that stakeholders’ needs are met in view of ever-changing requirements, even after the device has entered the marketplace. No matter what may happen during the life cycle of a product, the systems engineering approach is used every step of the way, from the Concept stage through to Utilization.
That ends our discussion on the systems engineering approach to medical device design. Next time we’ll begin unraveling some of the mysteries and misconceptions behind patenting inventions.
___________________________________________

|
Tags: buttons, commercial production, concept stage, cost reduction, electronic muscle stimulating device, engineering expert witness, forensic engineer, keypad, medical device design, medical device manufacturer, patenting inventions, premature wear, product life cycle, product service technician, Production Stage, service cost, stakeholder requirements, systems engineering, systems engineering approach, Utilization Stage
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability, Professional Malpractice | Comments Off on Systems Engineering In Medical Device Design – Utilization
Sunday, March 3rd, 2013
| Last time we began our look at the Production stage of systems engineering. We learned that cost reduction is a frequent component of this stage due to market fluctuations and ongoing stakeholder requirements to cut costs, and that savings can be made through substitution of plastic for metal parts. In fact, there are many faces to cost reduction. We’ll explore another of those today.
Cost reduction isn’t limited to material expense. Within the manufacturing process itself there are often ample opportunities for cost reduction. As an example let’s say we’re manufacturing a medical device known as a percussion therapy device on an assembly line employing 21 workers over three shifts. This line assembles 300 devices per day at a combined material and labor cost of $2,100 per unit.
Percussion therapy devices are frequently used within the medical setting as they perform the very important function of helping to dislodge mucous from patients’ lungs. As such, they are in high demand and the market for them competitive. In our scenario some stakeholders in the device’s manufacture, in this case sales and marketing managers, specify that a cost reduction of $200 per device is necessary to avoid losing ground to competitors.
In response to this directive, design engineers take a fresh look at the assembly process. They identified several bottlenecks at key junctures during which manual labor is involved. They note that due to the painstaking work required at these stages, production is slowed.
Assembly lines operate dynamically, meaning any disturbance in the flow of activities has vast repercussions down the line. Bottlenecks in flow slow production lines, just as they do traffic on key arteries. A tie-up on assembly lines equates to production delays, and these may lead to difficulty in filling customer orders. Impatient customers have been known to turn to competitors when their orders aren’t filled, and this translates to lost revenue to our manufacturer.
Next week we’ll see what manufacturing changes are employed to solve identified problems, and we’ll see how man’s best friend is not a dog, but a robot.
___________________________________________

|
Tags: assembly line, cost reduction, engineering expert witness, forensic engineer, manufacturing process, medical device design, metal parts, percussion therapy, plastic parts, Production Stage, stakeholder requirements, systems engineering
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability, Professional Malpractice | Comments Off on Systems Engineering In Medical Device Design – Production, Part 2
Monday, February 4th, 2013
| If you’ve been following along with our blog discussion on the systems engineering approach to medical device design, you should by now be convinced that instructions are important. In fact, the meticulous instructions produced during the manufacturing, operating, and maintenance phases of the Development stage are also crucial to later stages, that of Production and Utilization. Let’s finish up our discussion on the Development stage by taking a look at its final aspect, Preproduction.
The Preproduction aspect is instrumental to nipping potential problems in the bud before the medical devices go into actual production. In the initial Preproduction stages, systems engineers coordinate with the manufacturing and purchasing departments within the company as well as outside suppliers. The goal is to acquire all parts and equipment necessary to build a limited number of medical devices on the assembly line. Subjects such as preference in molded plastic components, motors, gears, pumps, springs, electronic components, circuit boards, wire, and tubing are discussed and agreed upon. Vendors are assessed with regard to their ability to produce parts when they are needed and that meet design specifications, satisfy quality requirements, and have costs that fall within budgetary constraints.
The assembly of Preproduction devices provides an opportunity for systems engineers to validate manufacturing and quality control instructions and assess the device design with regard to manufacturability, meaning, the extent to which devices can be manufactured with relative ease, at minimal cost, while maintaining maximum reliability. Devices manufactured during this aspect of the Development stage serve as a test. Are instructions clearly written? Do the device parts fit together as they should? Are parts strong enough to withstand the assembly process? Can the devices be assembled as quickly and easily as expected?
If the answer is “no” to any of these questions, then the device design and instructions must be returned to the design engineers and technical writers. Heads come together to rehash things and work out the bugs.
Next time we’ll continue with the Preproduction aspect of the Development stage to see how laboratory and field testing enables systems engineers to shake out any more bugs from the medical device design, operating instructions, and maintenance instructions.
___________________________________________

|
Tags: assembl, circuit boards, design engineers, Development Stage, electronic components, engineering expert witness, field testing, forensic engineer, gears, instructions, laboratory testing, maintenance, manufacturability, medical device, medical device manufacturing, molded plastic components, motors, preproduction device, Production Stage, pumps, quality requirements, reliability, specifications, springs, systems engineering in medical device design, technical writers, tubing, Utilization Stage, wire
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability, Professional Malpractice | Comments Off on Systems Engineering In Medical Device Design – Preproduction, Part I
Sunday, January 27th, 2013
| Last time we wrapped up our discussion on the development of quality control instructions for use during the Development stage of the systems engineering approach to medical device design. These instructions are used to guide quality control inspection and testing during the Production stage. Now let’s continue our discussion on the development of instructions for the Utilization stage, the stage when the medical device is actually put into operation by the end user.
In the systems engineering approach to medical device design, design engineers must work closely with technical writers, those responsible for writing operating and product service instructions. The objective here is to share the engineering staff’s intimate knowledge of the medical device’s design with the writers in order to ensure that instructions are clearly written, comprehensive, and follow a logical progression. Instructions must be written so as to be easily understood by lay people outside of the engineering profession and medical device industry, because most of the individuals using the device will be healthcare professionals and service technicians, individuals lacking a background in engineering or medical device development.
Instructions are not only meant for the eyes of end users. They are also subject to review by governmental agencies. This fact acts as a safeguard to ensure device compliance both with regulatory requirements and industry standards as regards cautions and warnings. For example, instructions may be required to caution the user to allow the device to warm up for a certain period of time before use to avoid patient discomfort when coming into contact with cold metal.
Instructions might also warn against a harmful interaction if the device is used in conjunction with other devices. For example, an electronic muscle stimulator may send electrical pulses into a patient’s body that can interfere with the operation of their heart pacemaker. No doubt this is something that the operator of the device and the patient would want to be informed of.
At this point our medical device design has been completed, and instructions and procedures written, but the Development stage is not yet complete. Next time we’ll continue our discussion on this stage to see how a systems engineering step helps us to be safe rather than sorry after full production of the device has begun.
___________________________________________

|
Tags: design engineers, Development Stage, engineering expert witness, forensic engineer, harmful interaction, heart pacemaker, maintenance instructions, medical device design, medical device industry, muscle stimulator, operating instructions, Production Stage, quality control inspection, quality control testing, regulations, service instructions, systems engineering approach, technical writer, Utilization Stage
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability, Professional Malpractice | Comments Off on Systems Engineering In Medical Device Design – Instructions, Part 4
Monday, January 7th, 2013
| You know those instructional inserts that come in just about everything you buy? If you’re lucky they’re a one-pager, showing a simple illustration of how your purchase works. But sometimes they’re multiple pages long, even approaching the length of a short story. This is often the case when the item in question is complex and contains many parts.
If you’re like some people you try to avoid reading these instructions, preferring to forge ahead to the assembly/usage stage as quickly as possible, and you’ve probably had your fair share of times that this approach didn’t pan out. You were forced to re-do things and crack open the instruction manual anyway. If the instructions were written clearly, you may have eventually come to regard them as indispensable.
Clearly written instructions are one of the desired end results of the Development stage of the systems engineering approach to medical device design that we’ve been discussing. These instructions flow naturally from the finalized detailed design which has been produced earlier in this stage. Instructions aid consumers in the assembly, usage, and maintenance of the device, making for a satisfied customer.
Instructions also aid in the efficient and proper manufacture of devices. Without them assembly personnel wouldn’t work as efficiently, and the end result might not be a desirable one. It’s easy for parts to end up where they don’t belong, adjustments to be off, etc. Just think about the last “assemble it yourself at home” project you were involved in.
The desired result is for instructions produced to be well defined and capable of instructing line assembly personnel in the actual construction of the medical device that takes place during the Production stage. Subjects such as parts identification, assembly procedures, and layout of assembly lines are discussed, all of which are needed to plan out the manufacturing process effectively. The objective is to manufacture the devices in a cost effective manner and with minimum probability of defects.
Next week we’ll continue our discussion on instructions, focusing on those that are produced during the Development stage that serve the purpose of guiding quality control technicians during the Production stage.
___________________________________________
|
Tags: assembly instructions, assembly lines, assembly procedures, detailed design, Development Stage, engineering expert witness, forensic engineer, manufacturing instructions, manufacturing process, medical device design, medical device design defect, Production Stage, quality control, systems engineering
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability, Professional Malpractice | Comments Off on Systems Engineering In Medical Device Design – Instructions, Part I