| Several years ago I was asked by power producers within the electric utility industry to write and then present a training course on the subject of coal power plant fundamentals. The finished product was a two day introductory course on the energy transformation process within a coal fired plant.
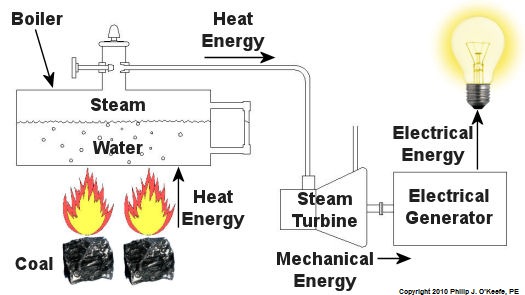
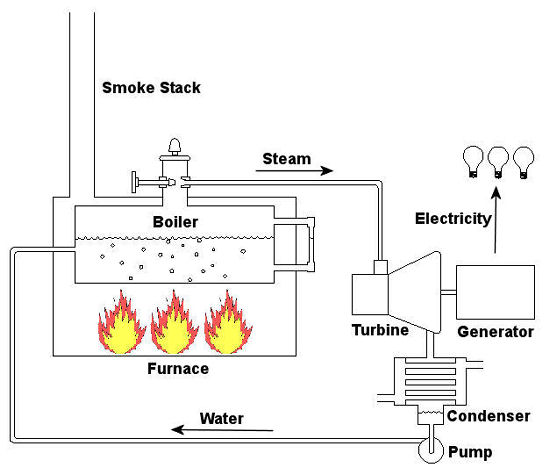
Since that time my seminar, entitled Coal Power Plant Fundamentals, has been presented to a variety of audiences, including Mirant Corporation, Platte River Power Authority, and Integrys Energy Group, Inc. Audience makeup has been diverse and has included equipment manufacturers, mining companies, power industry consultants, and regulatory agencies. This seminar, which I continue to present today in meeting rooms across the country, covers all major systems in a typical power plant, from coal handling when the coal first enters the plant, to its eventual end destination, the electrical switch yard which facilitates power transmission to customers. My Power Point presentation is embellished with ample illustrations, including photographs that I have taken during the course of my career and diagrams which I created using CAD, or Computer Aided Drawing software, one of which is featured below. In addition to the overhead slides, I provide a 150-page bound book which is distributed to seminar attendees. They use it to both follow along with my lecture and have a source of refresher material to take home with them. I’ve been told that having my illustrations in front of them makes a world of difference towards their understanding of the subject matter. The unique thing about my course is that it focuses on the simplified presentation of complex engineering concepts, much like my blogs do. Of course it always helps to have an engineering background or scientific background of sorts, but I wrote the course to accommodate understanding of the subject matter by individuals without any technical background. Accountants, salespersons, administrative staff, plant operating and maintenance workers, and journalists have all found the course to be easy to follow, interesting, and informative. So how do you get electricity from coal? To answer this question and give you a sampling of my seminar material let’s take a look at Figure 1. Figure 1 – The Coal Power Plant Energy Transformation Process Following along from left to right, the coal is first burned in order to transform the chemical energy which it contains into heat energy. That heat energy is then absorbed by water inside a nearby boiler, where it is converted into steam. The heat energy in the steam flows through a pipe into a steam turbine where it is again transformed, this time into mechanical energy that enables the turbine shaft to spin. The mechanical energy in the turbine is then transmitted by its shaft, enabling it to turn an electrical generator. And, finally, the mechanical energy is transformed by the generator into electrical energy for our usage. Simple process, right? Well, maybe, maybe not. My illustration certainly helped to simplify things, but there are a lot of details that were purposely omitted so as not to “muddy the waters.” It’s those details which have the potential to make things a lot more complicated, and next week we’ll begin to take a closer look at some of them. _____________________________________________ |
Posts Tagged ‘boiler’
Coal Power Plant Fundamentals
Sunday, January 23rd, 2011Pressurized Containers – Industrial Overpressure Devices
Sunday, October 17th, 2010| Perhaps you went out on a drive to enjoy a nice summer day. As you ventured into uncharted territory, you might have ended up in an industrial area. There, you noticed factories, chemical plants, and oil refinery complexes, each surrounded by a huge system of pipes and tanks. You might have considered it to be an eyesore, but if you’re an artist and engineer like I am, you might look at it as a form of art, composed of interesting shapes, colors, and patterns. No matter how you look at it, you can bet that there are at least a few pressurized containers in there.
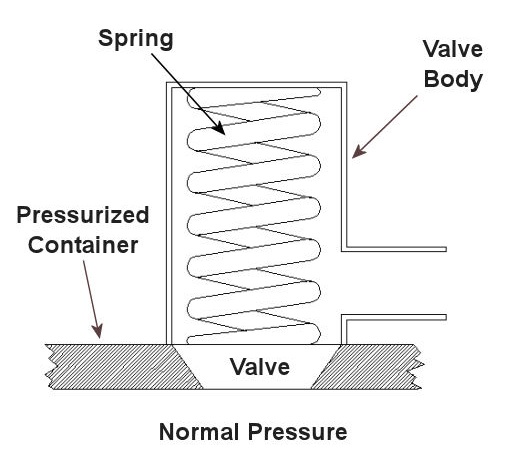
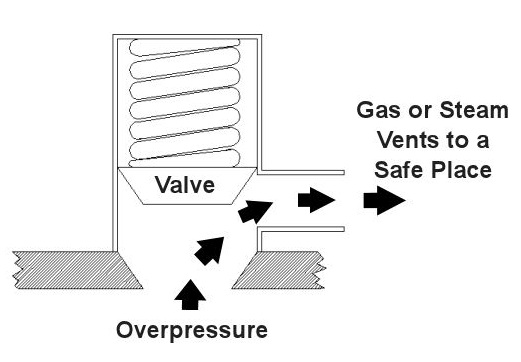
Last time we saw how something as seemingly harmless as a home water heater could become a dangerous missile if the pressure inside builds to the point where the tank ruptures. You can imagine what kind of explosive forces, steam, and chemicals would be unleashed into the surroundings if an industrial sized pressurized container failed due to overpressure. Let’s explore some other types of overpressure devices that are commonly used in industrial settings. One type of overpressure device is a safety valve. They are similar to a water heater relief valve, but they are generally used to relieve overpressure of gases and steam. How do they work? Basically, a safety valve is attached to the top of a pressurized container as shown in the cut away view in Figure 1 below. Figure 1 – A Basic Safety Valve In The Closed Position A powerful spring in the valve body is designed to force down on the valve and keep it closed if there is normal pressure inside the container. Once the pressure begins to rise to an unsafe level, it pushes up against the valve and overcomes the force of the spring. The valve opens, as shown in Figure 2 below, and the contents of the pressurized container are safely vented out to an area that is normally unoccupied by people. In case you’re wondering, safety valves are commonly used on pressurized storage tanks and boilers. Figure 2 – A Basic Safety Valve In The Open Position Another way to address the overpressure scenario is to employ a rupture disc. This is in fact a purposely constructed weak spot. It is intentionally built into a pressurized container and is designed so that it will fail when pressure starts to rise. In fact, this disc is designed to fail at a pressure point just below the pressure at which the container itself would fail. The disc is usually located within a vent pipe, which is in turn connected to the container. Should the disc rupture in an overpressure situation, the contents of the pressurized container will safely flow out of the vent pipe to a place normally unoccupied by people. The advantage of using a rupture disc is that they are made to safely release huge quantities of pressurized substances very quickly. The disadvantage in their usage is that they’re a one-time fix. That is, unlike relief or safety valves which may perform their function a multitude of times, a rupture disc is destroyed once it does its job. They are generally used in industrial settings where potential hazards are greater than at home, so once the rupture disc blows, the complete system generally undergoes a shut down so that the disc an be replaced before the pressurized container can be used again. Another option to pressure containment is the use of a fusible plug, usually constructed of a metal that will melt if the temperature within a pressurized container rises above a certain level. The metal plug melts, and excess pressure is vented through the aperture formed into a safe location. These are often used on locomotive boilers and compressed gas cylinders. Like rupture discs, fusible plugs are a one-time fix and must be replaced once they have done their job. Yet another option to pressure containment is to use a temperature limiting control. This category includes devices that monitor temperature and pressure within a pressurized container. If a dangerous situation should develop, the control system reacts, effectively reducing the pressure to prevent failure of the vessel. Automatic combustion control systems for boilers in electric utility power plants use temperature and pressure sensors to keep pressures within safe limits by regulating fuel and air input to the boiler. Next time we’ll cover the American Society of Mechanical Engineers (ASME) Boiler and Pressure Vessel Code (BPVC), which establishes rules governing the design, fabrication, testing, inspection, and repair of boilers and other pressurized containers. _____________________________________________ |
Coal Power Plants, Far From Perfect
Sunday, July 18th, 2010|
Did you know that even a perpetual motion machine will eventually come to a stop due to uncontrollable factors? Well, uncontrollable factors are at play in power plants, too. If you recall from our last article, heat rate is industry jargon for gauging how efficiently a coal-fired power plant is operating. We learned that heat rate can be affected by things like missing thermal insulation on pipes and equipment. Missing insulation is, of course, a thing that is under human control and easily corrected, but there are some things that affect heat rate that we just can’t do anything about. They’re called, appropriately enough, uncontrollable factors. Uncontrollable factors exist because anything devised and made by fallible humans who are beholden to the myriad laws of the universe cannot be 100 percent efficient. At their best utility coal fired power plants have an overall efficiency of between 30 and 40 percent. That means 60 to 70 percent of the energy available in the coal gets lost in the process of generating electricity. A terrible waste, right? And yet there’s nothing we can do to trim these losses until improvements in the present level of technology take place. Just as our ability to track microbes is dictated by the strength and accuracy of our magnifying equipment, so are we hampered by the tools we have at our disposal to deal with inefficiencies such as energy losses. So where does this energy get lost due to uncontrollable factors? The first and probably most obvious place to look is the smoke stack. Energy is also lost in three other ways: friction between equipment parts, auxiliary power consumption, and in a piece of equipment known as a condenser. Let’s look at each. In the most basic of terms, when coal is introduced into a power plant boiler it is combined with air and burned. This burning process releases heat energy, but it also forms gases that contain nitrogen and compounds like carbon monoxide and carbon dioxide. There’s also some water vapor formed by moisture in the coal and air. These gases and vapor absorb some of the heat energy released. To keep the combustion process going the gases and vapor must be removed from the boiler by powerful fans and sent up the smoke stack. Now, boilers are designed to absorb much of the heat energy from the gases and vapor that make their way to the stack, but they cannot possibly absorb it all. The result is that a significant amount of heat escapes up the smoke stack into the atmosphere along with the gases. Friction between parts is present everywhere in a power plant. It exists in the bearings on the shafts of motors, pumps, and steam turbines, slowing them down and hindering their operating capacity. Friction also exists where moving water and steam are present, impeding their ability to flow through piping systems. There is even friction working against the steam as it flows through parts in the turbine. Extra energy has to be expended to overcome this friction. This is energy that could be used to generate electricity. Now at some point in your life you’ve probably heard it said, “You need money to make money,” and this is very true. It takes a certain investment of resources to produce a profit-making enterprise. This investment principle holds true for the making of electricity, too. The bottom line is you need electricity to make electricity. Specifically, you have to use significant amounts of electricity to power machinery that is essential to move coal, air, combustion gases, and water through the process of making electricity in the power plant. This is called auxiliary power. It’s the electricity siphoned off by the various pieces of equipment in a power plant in its quest to generate electrical energy to be sold to customers. Another major factor at play in uncontrollable energy losses is in a piece of equipment integral to the very function of power plants: the condenser. It comes into play when water is boiled to make steam which then travels through the turbine, spinning its electrical generator and creating electric power. Unfortunately even the most efficient of steam turbines cannot use 100% of the heat energy coming at it from the steam. You see, after steam leaves the turbine, it’s turned back into water by a condenser so it can be sent back to the boiler to be turned into steam again. One of the reasons that this is done is so that the boiler does not have to be continuously filled with fresh, purified water. Water purification is necessary to keep minerals, seaweed, fish scales, and other nasty things from clogging up and damaging the boiler and steam turbine, and purified water is not as readily available as, say, lake water. The condenser acts as a heat exchanger that is hooked up to the steam turbine exhaust. It has tubes inside of it in which cold water flows, water which is drawn in from a nearby body of water, most often a river or lake. As steam blows across the outside of the cold water tubes in the condenser, it gives up its remaining heat energy and condenses into water again, then it is returned to the boiler to repeat its journey. The river water within the tubes of the condenser flows back into the river, carrying with it the heat energy removed from the steam. That wraps up our discussion about coal power plant efficiency. Next time we’ll discuss a new topic: coal fired power plant furnace explosions. _____________________________________________ |
Fossil Fuel, From Friend to Foe
Sunday, June 27th, 2010|
Did you ever have someone you considered to be a great friend and then things suddenly went bad between you? One day you’re chums and then the magic fades, soon to disappear? Sound like some marriages you’ve heard about? Well, it wasn’t too long ago that coal was considered to be America’s affordable answer to our fuel needs. It was a friend of grand proportions, there when you needed it. It remains an abundant resource, so abundant in fact that according to the US Energy Information Administration (EIA) we are sitting on coal reserves so vast they can provide us with sufficient energy to get us through the next 250 years at current rates of consumption. It was for these reasons that electric utilities decided decades ago to use coal as the primary source of fuel to generate electricity, and as it stands now just over 50% of our electrical energy is generated by burning coal. So how did coal go from being friend to foe? Well, just as when you’ve known someone for awhile their “baggage” becomes more apparent, it eventually became apparent to Americans that burning coal comes with some nasty baggage of its own, known as byproducts. These unwelcome components of the burning/oxidation process were found in the plumes of smoke that billowed out of power plants’ smokestacks. So just what are these byproducts? Well, some of it is the same stuff that’s left over at the bottom of your barbecue grille after a cookout, and some of it comes with scientific names like sulfur dioxide (SO2), nitric oxide (NO), and nitrous oxide (N2O). Let’s look at these in more detail. Ash is the residue that’s left behind after coal is burned. Fly ash is a type of ash that is made up of some very light particles and it can get carried away by the hot gases coming off the fire in a power plant boiler. Some of those particles manage to leave the smokestack and enter the environment. Sulfur dioxide, or SO2, is formed when the sulfur in coal combines with oxygen in the air during burning. When the SO2 leaves the smokestack, it can combine with moisture in the atmosphere to form acid rain. Most of us know what acid rain is, but for those that don’t, acid rain does things like rust metal, dissolve marble monuments, and in general disrupt the balance of Earth’s eco systems. Nitric oxide, NO, and nitrous oxide, N2O, are chemical compounds composed of nitrogen and oxygen that fall into the group commonly referred to as NOx, pronounced “knocks.” NOx is formed when nitrogen and oxygen in the air combine at the high temperatures released when coal is burned inside power plant furnaces. NOx is bad because its compounds are key ingredients in the formation of both acid rain and smog. Over the last thirty years emissions of these byproducts have come under increasing scrutiny by federal and state regulators in their quest to curb them and their impact on our environment. As a result, electric utilities have had to comply with ever-tightening regulations. To comply, coals with lower sulfur content have been used, often brought in over very long distances from mines in the US and even foreign countries like Columbia. Utilities have also been installing expensive pollution control equipment in their coal fired power plants. But these changes make operations more expensive, eating into the utilities’ profits. Now we may not like the idea of utilities earning a profit, but this is a necessary reality to some extent in order to keep their business solvent. They’re not in it for the fun of it, after all. And I’m sure you guessed by now that the net result of the regulatory agencies’ mandates is that our electric bills just keep escalating. Now much of what lies behind the current unfavorable status of coal powered plants is that when operating on our native soil they have high visibility. We don’t like to be reminded of the negatives that accompany the production of energy. Put that same plant in another faraway country and the byproducts cease to be an issue. It’s happening over there after all, and we don’t have to be confronted with it. We neglect to remind ourselves that the earth’s atmosphere is for the most part a contained unit, and that means that what happens there is happening here, whether there happens to be on the other side of the globe or not. Next week we’ll continue our explorations into coal, examining the impact of the low sulfur variety on electric utility power generation. _____________________________________________ |
Thermodynamics In Mechanical Engineering, Part II, Power Cycles
Sunday, December 13th, 2009|
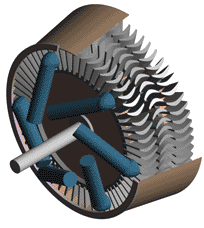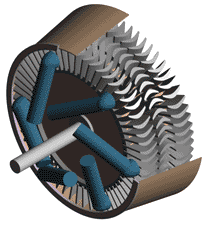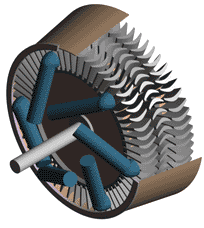
Last time we talked about some general concepts in an area of mechanical engineering known as thermodynamics. In this week’s article we’ll narrow our focus a bit to look at a part of thermodynamics that deals with power cycles. One mammoth example of a power cycle can be found in a coal-fired power plant. You can’t help but notice these plants with their massive buildings, mountains of coal, and tall smoke stacks. They’ve been getting a lot of negative press lately and are a central focus of the debate on global warming, but most people have no idea what’s going on inside of them. Let’s take a peek. Figure 1 – A Coal-Fired Power Plant A power plant has one basic function, to convert the chemical energy in coal into the electrical energy that we use in our modern lives, and it’s a power cycle that is at the heart of this conversion process. The most basic power cycle in this instance would include a boiler, steam turbine, condenser, and a pump (see Figure 2 below). Figure 2 – A Basic Power Cycle When the coal is burned in the power plant furnace, its chemical energy is turned into heat energy. This heat energy and the boiler are enclosed by the furnace so the boiler can more efficiently absorb the heat energy to make steam. A pipe carries the steam from the boiler to a steam turbine. Nozzles in the steam turbine convert the heat energy of the steam into kinetic energy, making the steam pick up speed as it leaves the nozzles. The fast moving steam transfers its kinetic energy to the turbine blades, causing the turbine to spin, much like a windmill (see Figure 3 below). Figure 3 – The Inner Workings of a Steam Turbine The spinning turbine is connected by a shaft to a generator. The turbine works to spin the generator and thus produces electricity. After the energy in the steam is used by the turbine, it goes to the condenser, whose job it is to convert the steam back into water. To accomplish this, the condenser uses cold water, say from a nearby lake or river, to cool the steam down until it converts from a gas back to a liquid, that is, water. This is why power plants are normally found adjacent to a body of water. After things are cooled down, the pump gets to work, pushing the condensed water back into the boiler where it is once again turned into steam. This power cycle keeps repeating itself as long as there is coal being burned in the furnace, the plant equipment is functioning properly, and electrical energy flows out of the power plant. Thermodynamics sets up an energy accounting system that enables mechanical engineers to design and analyze power cycles to make sure they are safe, reliable, efficient, and economical. When all is said and done, a properly designed power cycle transfers as much heat energy as possible from the burning coal on one end of the cycle to meet the requirements for electrical power on the other end of the cycle. As was mentioned in last week’s blog, nothing is 100% efficient. Next time we’ll learn about being cool. No, I’m not going to talk about the latest cell phone gadget or who’s connected on Facebook. We’ll be covering refrigeration cycles. _________________________________________________________________ |