|
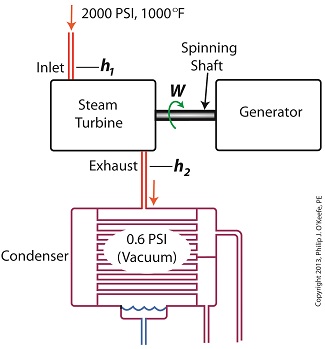
Last time we ran our basic power plant steam turbine without a condenser. In that configuration the steam from the turbine exhaust was simply discharged to the surrounding atmosphere. Today we’ll connect it to a condenser to see how it improves the turbine’s efficiency. As discussed in a previous blog, enthalpy h1 is solely dependent on the pressure and temperature at the turbine inlet. For purposes of today’s discussion, turbine inlet steam pressure and temperature will remain as last time, with values of 2,000 lbs PSI and 1000°F respectively, and calculations today will be based upon those values. So to review, the inlet enthalpy h1 is, h1 = 1474 BTU/lb If the condenser vacuum exists at a pressure of 0.6 PSI, a realistic value for a power plant condenser, then referring to the steam tables in the Van Wylen and Sonntag thermodynamics book, we find that the enthalpy h2 will be, h2 = 847 BTU/lb and the amount of useful work that the turbine can perform with the condenser in place would therefore be, W = h1 – h2 = 1474 BTU/lb – 847 BTU/lb = 627 BTU/lb So essentially with the condenser present, the work of the turbine is increased by 168 BTU/lb (627 BTU/lb – 459 BTU/lb). To put this increase into terms we can relate to, consider this. Suppose there’s one million pounds of steam flowing through the turbine each hour. Knowing this, the turbine power increase, P, is calculated to be, P = (168 BTU/lb) ´ (1,000,000 lb/hr) = 168,000,000 BTU/hr Now according to Marks’ Standard Handbook for Mechanical Engineers, a popular general reference book in mechanical engineering circles, one BTU per hour is equivalent to 0.000393 horsepower, or HP. So converting turbine power, P, to horsepower, HP, we get, P = (168,000,000 BTU/hr) ´ (0.000393 HP/BTU/hr) = 66,025 HP A typical automobile has a 120 HP engine, so this equation tells us that the turbine horsepower output was increased a great deal simply by adding a condenser to the turbine exhaust. In fact, it was increased to the tune of the power behind approximately 550 cars! What all this means is that the stronger the vacuum within the condenser, the greater the difference between h1 and h2 will be. This results in increased turbine efficiency and work output, as evidenced by the greater numeric value for W. Put another way, the turbine’s increased efficiency is a direct result of the condenser’s vacuum forming action and its recapturing of the steam that would otherwise escape from the turbine’s exhaust into the atmosphere. This wraps up our series on the power plant water-to-steam cycle. Next time we’ll use the power of 3D animation to turn a static 2D image of a centrifugal clutch into a moving portrayal to see how it works. ________________________________________ |
Posts Tagged ‘coal power plant’
How Condensers Increase Efficiency Inside Power Plants
Wednesday, December 4th, 2013Enthalpy and Steam Turbines
Thursday, November 14th, 2013|
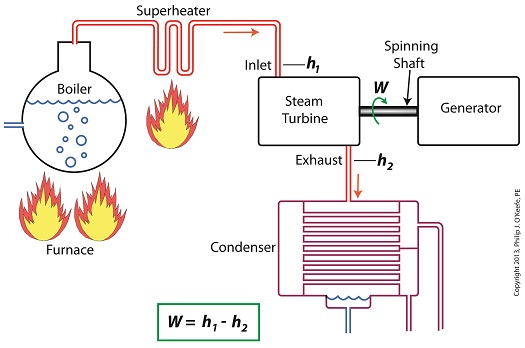
Last time we learned how the formation of condensate within a power plant’s turbine results in a vacuum being created. This vacuum plays a key role in increasing steam turbine efficiency because it affects a property known as enthalpy, a term used to denote total heat energy contained within a substance. For the purposes of our discussion, that would be the heat energy contained within steam which flows through the turbine in a power plant. The term enthalpy was first introduced by scientists within the context of the science of thermodynamics sometime in the early 20th Century. As discussed in a previous blog article, thermodynamics is the science that deals with heat and work present within processes. Enthalpy is a key factor in thermodynamics, and is commonly represented in engineering calculations by the letter h and denoted as, h = u + Pv where u is the internal energy of a substance, let’s say steam; P is the pressure acting upon a specific volume, v, of the steam; and P and v are multiplied together. Pressure is force per unit area and is measured in psi, pounds per square inch. For the purposes of our discussion, it’s the amount of pressure that steam places on pipes containing it. Looking at the equation above, simple math tells us that if we increase the pressure, P, the result will be an increase in enthalpy h. If we decrease P, the result will be a decrease in h. Now, let’s see why this property is important with regard to the operation of a steam turbine. When it comes to steam turbines, thermodynamics tells us that the amount of work they perform is proportional to the difference between the enthalpy of the steam entering the turbine and the enthalpy of the steam at the turbine’s exhaust. What is meant by work is the act of driving the electrical generator, which in turn provides electric power. In other words, the work leads to a useful outcome. This relationship is represented by the following equation, W = h1 – h2 In terms of the illustration below, W stands for work, or potential for useful outcome of the turbine/generator process in the form of electricity, h1 is the enthalpy of the steam entering the inlet of the turbine from the superheater, and h2 is the enthalpy of the steam leaving at the turbine exhaust. We’ll discuss the importance of enthalpy in more detail next week, when we’ll apply the concept to the work output of a steam turbine.
________________________________________ |
Vacuum in a Power Plant Condenser
Tuesday, November 5th, 2013|
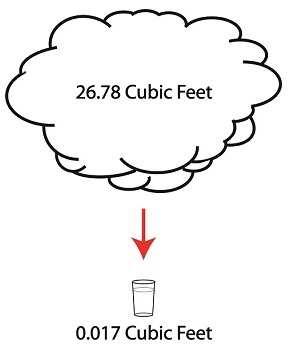
Last time we discussed the key functions of the make-up valve in the power plant water-to-steam cycle. Today we’re going to talk about a vacuum. No, not the kind you use around the house, the kind that’s created by the condenser inside a power plant. As discussed previously, the condenser is a piece of equipment that turns turbine exhaust steam back into water. The water that’s formed during this process is known as condensate, and its density is higher than that of the steam it shares space with inside the condenser. That difference in density is what creates the vacuum inside the condenser vessel. Put another way, the increase in density along with the condenser’s airtight design prevent air from rushing in from outside to occupy any of the space inside the condenser, a desirable condition from an efficiency standpoint. But to understand how all this works we’ll first have to gain an understanding of what is meant by density. A textbook would define it as the mass of a substance divided by the amount of space that that substance occupies. Let’s take steam and water for example. One pound of steam at 212°F forms a vapor cloud that occupies 26.78 cubic feet of space. If we condensed that pound of steam back into water at the same temperature, it would just about fit into a 16 ounce glass and occupy a mere 0.017 cubic feet. The huge difference in their volumes is due to the fact that steam contains more than five times the heat energy that unheated water does. That energy makes the molecules in a cloud of steam more active, causing them to collide against each other with great force, spread apart, and occupy a larger space. If you’re wondering what change in density has to do with vacuum in the condenser, allow me to offer an analogy. Ever canned any produce, like tomatoes, in glass jars to over-winter? Not likely, as this once common survival tactic has nearly become a lost art. But the vacuum created inside the condenser is much like the vacuum created within a mason jar during canning. Inside the glass mason jar, a small space is intentionally left between the tomatoes and lid. During the process of boiling, or heat sterilization, this space fills with steam. Then during cooling the trapped steam condenses into water. This condensation creates the vacuum that sucks down on the jar’s lid, giving it an airtight seal, a condition which won’t allow bacteria to grow on our canned foods. You see, like us bacteria need oxygen to live, but thanks to the vacuum inside our cooked mason jar no air containing oxygen will remain inside to harbor it. Next time we’ll continue our discussion on vacuum to see how it’s used to increase a steam turbine’s efficiency.
________________________________________ |
How A Power Plant Condenser Works, Part 2
Sunday, October 6th, 2013|
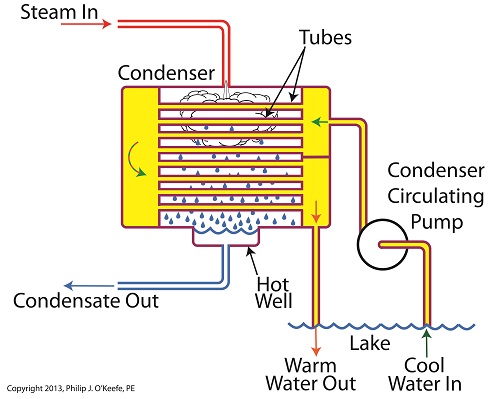
Winter is fast approaching. Imagine living in a house without insulation. Now imagine your heating bill, which will be high due to the tremendous amount of heat loss. Energy is a precious resource, no matter how it’s produced, and its conservation within a power plant’s steam/water cycle is of vital importance. Last time we learned about the transfer of heat energy within a power plant’s condenser, where some of the heat energy contained within its steam is absorbed by the cool lake water contained inside its tubes. Steam is continuously flowing into the condenser from the steam turbine, so it’s essential for the circulating pump to keep a fresh supply of lake water flowing through the condenser’s tubes in an effort to keep temperatures under control. The compensating action that’s provided by the cool lake water flowing within the tubes, represented by green arrows in the illustration, keeps the temperature inside the tubes from rising and becoming equal to the steam’s temperature outside of them. If the flow of cool water through the tubes were to stop and the temperatures inside and outside the tubes become equal, the water contained inside the tubes would boil off to steam, resulting in the tubes bursting and a wrecked condenser. After absorbing heat energy from the surrounding steam, the warmed lake water within the tubes follows a circuitous path through the tubing, eventually emptying out into the lake. The orange arrows in the illustration show this path. Okay, with this warm water entering the lake, doesn’t that harm the eco system? Actually its impact is negligible. You see, the temperature of the lake water leaving the condenser is only about 10°F higher than when it was pumped from the lake. Add this to the fact that the volume of water contained within a lake is huge in comparison to the small amount of warmed water being returned to it. Next week we’ll see how the loss of heat energy affects the steam, and how an important part of the condenser known as the hot well comes into play. ________________________________________ |
How A Power Plant Condenser Works, Part 1
Wednesday, October 2nd, 2013|
Last time we began our discussion on power plant inefficiencies and indentified a major contributor, the heat energy dispelled into the atmosphere through the turbine exhaust. Today we’ll see how a piece of equipment known as the condenser comes into play to deal with this problem. Let’s see how it works. First, water from our plant’s water source, say a nearby lake, is siphoned into the condenser circulating pump, which delivers it to the condenser. This lake water path appears in yellow. You’ll notice that the lake water follows a circuitous path from the lake, through the condenser circulating pump, then the condenser tubes, until finally it is returned to the lake. Now the cool lake water, denoted by green arrows, is made to pass through the condenser’s many tubes, while steam from the turbine exhaust surrounds them. The tubes keep the lake water segregated from the cloud of steam surrounding them inside the condenser vessel. In other words, the lake water’s path is a closed system, never coming into direct physical contact with the surrounding steam. What’s happening inside our condenser is demonstrative of a fundamental principle of thermal engineering, that is, that hot will travel in the direction of cold. More specifically, within our condenser the heat energy in the steam cloud surrounding the condenser tubes will be attracted to the cool lake water contained within the tubes. This causes the heat energy contained within the steam to leave it, and get absorbed by the cool lake water flowing within the tubes. We’ll begin to find out how these dynamics influence what’s happening with our water-to-steam power plant cycle next time.
________________________________________ |
Superheater Construction and Function
Sunday, September 15th, 2013|
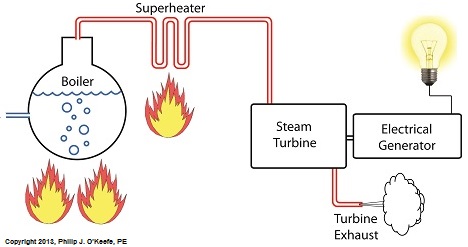
Power plants produce electrical energy for consumers to use, whether at home or for business, that’s obvious enough, but did you know that in order to produce that electrical energy they must first be supplied with heat energy? The heat energy that power plants crave comes from a fuel source, such as coal, oil, or natural gas, by way of a burning process. Once the heat energy is released from the coal through burning, it’s transported into a steam turbine by way of superheated steam, which is supplied to it by a piece of equipment named, appropriately enough, a superheater. So what is a superheater and how does it function? Take a look at the illustration below. The superheater looks like a W. It’s actually a cascading array of bent steam pipe, situated above a source of open flames which are produced by the burning of a fuel source. A photo of an actual superheater is shown below. So how many bends are in a superheater? Enough to fill the needs of the particular power plant it is supplying energy to. Since all power plants are designed differently, we’ll keep things in general terms. The many bends in the superheater’s pipes form a circuitous path for steam to flow as it follows a path from the boiler to the steam turbine. The superheater’s unique construction gives the steam flowing through it maximum exposure to heat. In other words, the bends increase the time it takes for the steam to flow through the superheater. The more bends that are present, the longer the steam will be exposed to the flame’s heat energy, and the longer that exposure, the more heat energy that is absorbed by the steam. Superheating routinely results in temperatures in excess of 1000°F. This superheated steam is laden with abundant heat energy which will keep the steam turbine spinning and the generator operating. The net result is millions of watts of electrical power. As we learned in a previous blog, the superheater is designed to provide the turbine with sensible heat energy to prevent steam from completely desuperheating, which would result in dangerous condensation inside the turbine. The newly added superheater is a major improvement to a power plant’s water-to-steam cycle, but there’s still plenty of waste and inefficiency in the system, which we’ll discuss next week.
________________________________________ |
Superheating, Part 2
Sunday, August 25th, 2013|
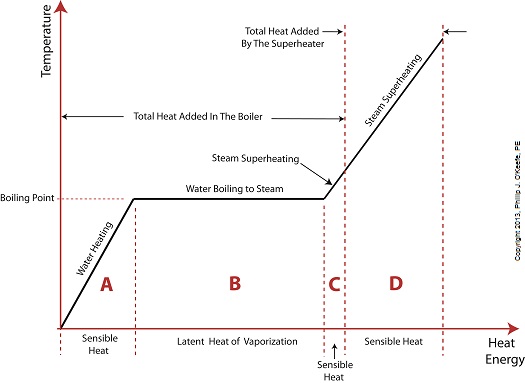
Last time we added a piece of equipment called a superheater, positioned between the boiler and steam turbine, to our basic electric utility power plant steam and water cycle. Its addition enables a greater and more consistent supply of heat energy to the steam which powers the turbine. How much more? Let’s look at Figure 1 to get an idea. Figure 1
You may have noticed that our illustration lacks numerical representation. That’s because power plants are designed differently, depending on fuels used and power output required. So unless we’re talking about a particular power plant, number values would be impractical. For example, I could specify a boiling point of 596°F at 1,500 pounds per square inch (PSI), and a superheater outlet temperature of 1,050°F at 1,200PSI, and I could make note of esoteric things like enthalpy (British Thermal Units per pound mass) values on the Heat Energy axis. But to facilitate our discussion we’ll keep things simple and focus on the general process. Figure 1 shows in phase D the additional heat energy being added to the steam, thanks to the superheater. This is significantly more than had been added by the boiler alone, as represented by phase C. The turbine consumes heat energy added in phases C and D and converts it into mechanical energy to drive the generator, resulting in electrical energy being provided to consumers in the most energy efficient way possible. But increasing power output and efficiency isn’t the superheater’s only job. The heat it adds during phase D ensures the turbine’s safe operation when it’s cranking at full capacity, as represented by the superheated steam zones of phases C and D. Next week we’ll discover how the superheater prevents a destructive process known as condensing from occurring inside the turbine. ________________________________________ |
Heat Energy Within the Power Plant—The Power Behind the Turbines
Monday, July 29th, 2013|
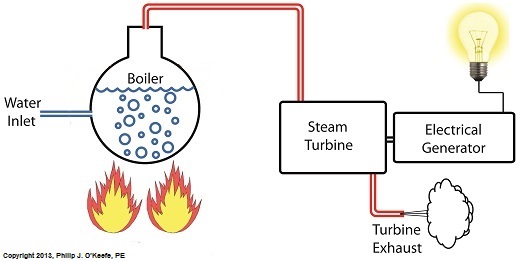
Last time we discovered that the boiling point of water varies. It’s dependent upon the amount of pressure exerted on its surface, which varies due to a variety of reasons, including where it is in relation to sea level. Before we see what happens under higher than atmospheric pressures, such as exist in an electric utility power plant boiler, let’s cover some basics. In the power plant, water is heated in a boiler specifically to produce steam, unlike our tea kettle where the primary purpose is to produce hot water. The steam produced is used to spin turbine generators, which in turn generate electricity, as I explained in a previous blog on steam turbines. Unlike a tea kettle, which is open to the atmosphere on your kitchen stove, the boiler in a power plant is an enclosed, reinforced steel vessel. See illustration below. The reinforced steel boiler vessel is designed to withstand great internal pressure as temperatures rise within. In addition to providing a safety feature, the enclosed space provides a sheltered environment for collecting steam so it can later be put to use spinning power generating turbines down the line. In other words, surface water inside the boiler is closed off from the surrounding atmosphere, allowing its internal pressure to build for our specific purposes. As heat energy is added to water within the boiler, the water boils and steam bubbles break out from its surface, filling the empty space above the surface with pressurized steam. This steam will try to expand here, but it can’t, because it’s being constrained by the reinforced steel vessel within which it is enclosed. Instead, steam pressure builds up on the surface of the water inside the boiler until it is high enough to be released through an attached pipe which is connected to a nearby turbine. We’ll talk more about this pent-up energy and how it is put to use within the power plant in next week’s blog. ___________________________________________
|
Coal Fired Boiler Explosions
Sunday, July 25th, 2010|
Try this for a tongue-twister: Coal fired electric utility power plant boiler… If you’ve been reading along with us for the last couple of weeks, you now have a pretty good idea of what these are and what they do. These boilers are contained within furnaces in coal fired power plants. The furnace’s job is to combine coal and air to create a combustion process. It is like a big, insulated enclosure that keeps the heat energy from the combustion process from escaping before it can be absorbed by the water and steam in the boiler tubes. The heat energy is then funneled to the steam turbine to spin an electrical generator, creating the energy which will eventually find its way into our homes and businesses. During the operation of the boiler, coal and air must be introduced into the furnace at carefully measured rates to maintain a proper fuel-to-air ratio which will enable the release of heat energy from the coal at a safe, controlled rate. Fuel-air ratio is the amount of coal entering the furnace divided by the amount of air entering the furnace. If this ratio isn’t precisely maintained, conditions may be right for an explosion to occur. Specifically, the ratio has to fall within an “explosive range.” Once within this range, all that is needed is an ignition source, such as hot ash, or even mere static electricity, and the result may be a furnace explosion. There are certain times at which furnace explosions are more likely to occur than others, such as when the boiler is being started, operated at less than full capacity, or shut down. When a furnace explodes, a pressure wave moves out from the center of the blast. This pressure wave will bear up against the sides of the furnace with great force, and if the pressure is high enough the sides of the furnace, which are made of heavy steel components, will actually bend and split open. Boiler tubes may even rupture, releasing high pressure steam and water into the power plant and furnace. At the very least, the boiler will be down for expensive repairs and no electricity can be produced by its turbine generator. This down time can last for many months and results in lost revenue to the energy producer. Aside from an explosive fuel-to-air ratio, there are other potential causes of furnace explosions. For example, poor coal quality can lead to incomplete combustion, or the flame going out completely, encouraging unburned coal particles to settle and accumulate in the furnace. The accumulation of coal can grow to the point where it forms an explosive mixture when combined with the right amount of air. So how can boiler explosions be prevented? The National Fire Protection Association (NFPA) looked into the problem and developed an industry standard. This standard is known as NFPA 85, Boiler and Combustion Systems Hazards Code. Its purpose is to contribute to operating safety and prevent uncontrolled fires, explosions, and implosions of coal fired boilers. NFPA 85 lays out guidelines to follow when designing, building, and operating boiler fuel handling systems, air handling systems, and combustion control systems. Following its guidelines will certainly significantly decrease the probability of explosions occurring. Another means of explosion prevention includes implementing a boiler operator training program. These enable attendees to better understand operating procedures and equip them with the knowledge to safely control the combustion process, particularly when a furnace explosion is most likely to occur. This training can be done with a combination of classroom instruction along with time on a simulator and may be followed up with hands-on training in the plant itself. Lastly, boiler explosions can be prevented by implementing an effective inspection and maintenance program to locate and repair or replace boiler components, averting the possibility of a potential disaster occurring. Things such as check lists can be used to ensure that nothing is missed. This is a strategy that all pilots must use before starting their planes, and it is now being used in hospitals as well to cut back on the rate of patient infection due to carelessness on the part of hospital staff. Hey, we’re all human, and humans are not perfect. But remember that an ounce of prevention is truly worth a pound of cure, and then some. A properly placed check on the list could mean lives will be saved. _____________________________________________ |