|
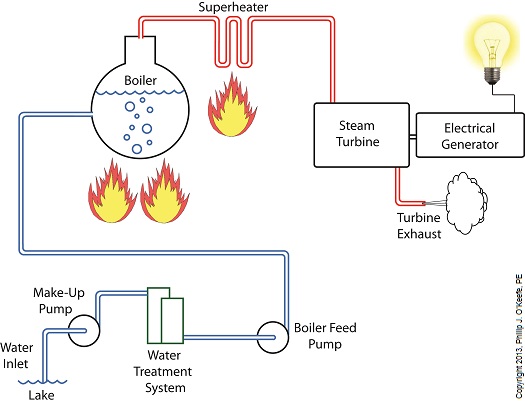
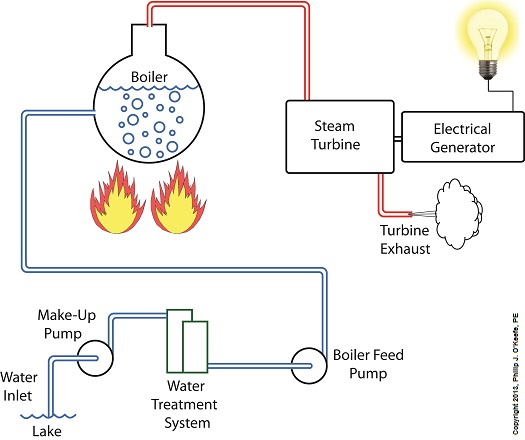
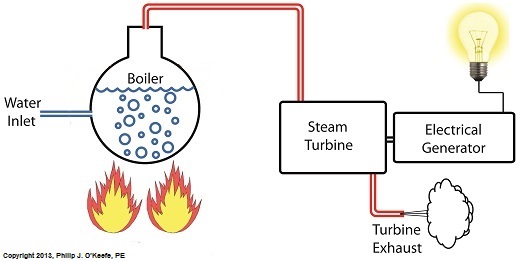
Last week we identified some inefficiencies in our water to steam power plant energy cycle. The superheater addressed some of these concerns, but not others. Our illustration discloses one of these wasteful areas to be coming from the turbine exhaust. That’s energy laden steam being expelled into the surrounding atmosphere! It’s the same heat energy that was produced in the boiler when water was transformed into steam. It came from burning fuels like coal, natural gas, and oil, all expensive and precious natural resources. In its present configuration the power plant will work, but because steam is being continually dispersed into the atmosphere, it must continually be replenished. The key ingredient, water, must be drawn into the power plant from a nearby source, treated for contaminants, then fed into the boiler to make up for lost steam. That wastes both water and energy, because the make-up pump, which draws water from the lake for treatment, (thus “making up” for spent water), is continuously operating, resulting in excessive wear and tear and increased operating costs. Fortunately, power plant engineers have devised methods to correct these inefficiencies. They’ve come up with a clever means of recapturing exhaust steam, thus enabling it to recycle within the system. Next week we’ll see how this is accomplished with a piece of equipment called a condenser.
________________________________________ |
Posts Tagged ‘heat energy’
Superheater Construction and Function
Sunday, September 15th, 2013|
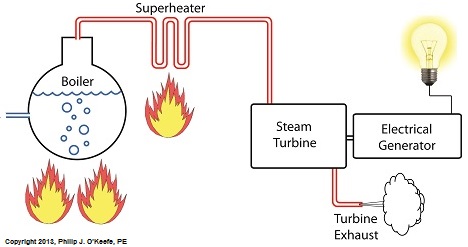
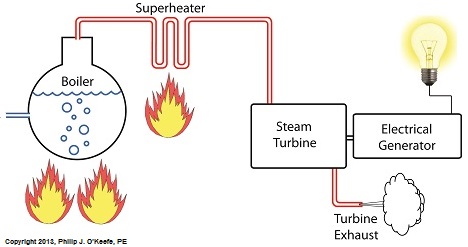
Power plants produce electrical energy for consumers to use, whether at home or for business, that’s obvious enough, but did you know that in order to produce that electrical energy they must first be supplied with heat energy? The heat energy that power plants crave comes from a fuel source, such as coal, oil, or natural gas, by way of a burning process. Once the heat energy is released from the coal through burning, it’s transported into a steam turbine by way of superheated steam, which is supplied to it by a piece of equipment named, appropriately enough, a superheater. So what is a superheater and how does it function? Take a look at the illustration below. The superheater looks like a W. It’s actually a cascading array of bent steam pipe, situated above a source of open flames which are produced by the burning of a fuel source. A photo of an actual superheater is shown below. So how many bends are in a superheater? Enough to fill the needs of the particular power plant it is supplying energy to. Since all power plants are designed differently, we’ll keep things in general terms. The many bends in the superheater’s pipes form a circuitous path for steam to flow as it follows a path from the boiler to the steam turbine. The superheater’s unique construction gives the steam flowing through it maximum exposure to heat. In other words, the bends increase the time it takes for the steam to flow through the superheater. The more bends that are present, the longer the steam will be exposed to the flame’s heat energy, and the longer that exposure, the more heat energy that is absorbed by the steam. Superheating routinely results in temperatures in excess of 1000°F. This superheated steam is laden with abundant heat energy which will keep the steam turbine spinning and the generator operating. The net result is millions of watts of electrical power. As we learned in a previous blog, the superheater is designed to provide the turbine with sensible heat energy to prevent steam from completely desuperheating, which would result in dangerous condensation inside the turbine. The newly added superheater is a major improvement to a power plant’s water-to-steam cycle, but there’s still plenty of waste and inefficiency in the system, which we’ll discuss next week.
________________________________________ |
Condensation Inside the Steam Turbine
Sunday, September 8th, 2013|
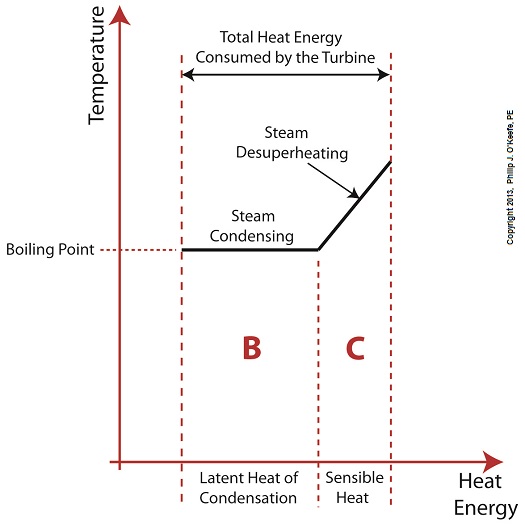
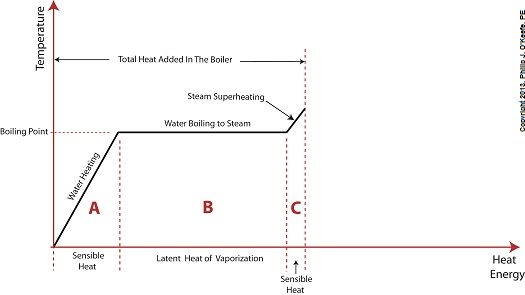
Did you know that water droplets traveling at high velocity can take on the force of bullets? It can happen, particularly within steam turbines at a power plant during the process of condensation, where steam transforms back into water. The last couple of weeks in this blog series we’ve been talking about the steam and water cycle within electric utility power plants, how heat energy is added to water during the boiling process, and how turbines run on the sensible heat energy that lies within the superheated steam vapor supplied by boilers and superheaters. We learned that without a superheater there is a very real possibility that the steam’s temperature can fall to mere boiling point. When steam returns to boiling point temperature an undesirable situation is created. The steam begins to condense into water within the turbine. To understand how this happens, let’s return to our graph from last week. It illustrates the situation when there’s no superheater present in the power plant’s steam cycle. Figure 1
After consuming all the sensible heat energy in phase C in Figure 1, the only heat energy which remains available to the turbine is the latent heat energy in phase B. If you recall from past blog articles, latent heat energy is the energy added to the boiler water to initiate the building of steam. As the turbine consumes this final source of heat energy, the steam begins a process of condensation while it flows through the turbine. You can think of condensing as a process that is opposite to boiling. During condensation, steam changes back into water as latent heat energy is consumed by the turbine. When the condensing process is in progress, the temperature in phase B remains at boiling point, but instead of pure steam flowing through the turbine, the steam will now include water droplets, a dangerous mixture. As steam flows through the progressive chambers of turbine blades, more of its latent heat energy is consumed and increasingly more steam turns back into water as the number of water droplets increases. Figure 2 – Water Droplets Forming in the Turbine
The danger comes in when you consider that the steam/water droplet mixture is flying through the turbine at hundreds of miles per hour. At these high speeds water droplets take on the force of machine gun bullets. That’s because they act more like a solid than a liquid due to their incompressible state. In other words, under great pressure and at high speed water droplets don’t just harmlessly splash around. They hit hard and cause damage to rapidly spinning turbine blades. Without a working turbine, the generator will grind to a halt. So how do we supply the energy hungry turbine with the energy contained within high temperature superheated steam in sufficient quantities to keep things going? We’ll talk more about the superheater, its function and construction, next week.
________________________________________ |
Desuperheating in the Steam Turbine
Monday, September 2nd, 2013|
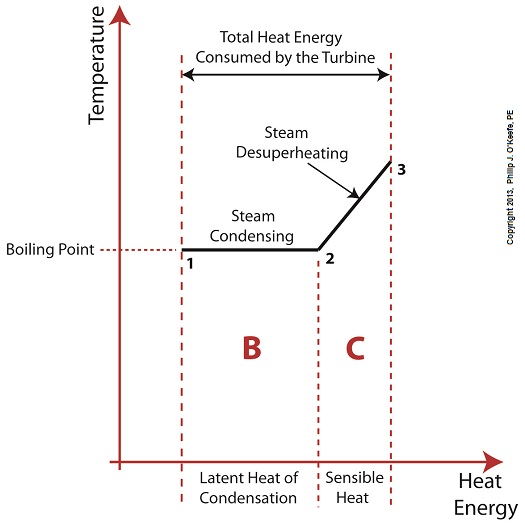
Last time we learned that the addition of a superheater to the electric utility power plant steam cycle provides a ready supply of high temperature steam, laden with heat energy, to the turbine, which in turn powers the generator. But this isn’t its only job. One of the superheater’s most important functions is to regulate the ongoing process of desuperheating that takes place as the turbine consumes heat energy. To understand this, let’s see what takes place if the superheater were to be removed from its position between the boiler and turbine. Figure 1
Without the superheater, the only available remaining source of sensible heat energy to the turbine would come from the meager amount present in phase C steam as shown in Figure 1. If you’ll recall from a past blog, the sensible heat energy contained in superheated steam is the best source of energy for a steam turbine, because it’s able to keep it operating most efficiently. As the turbine consumes the heat energy in phase C, starting at point 3 and continuing to point 2, the steam it’s consuming is in the process of desuperheating, as evidenced by the downward slope between the two points. Desuperheating is an engineering term which means that as sensible heat energy is removed from the steam due to its use by the turbine, there will be a resulting drop in steam temperature. And if this process were to continue without the compensatory function provided by the addition of a superheater to the steam cycle, the steam’s temperature would eventually return to mere boiling point, at point 2. This is an undesirable thing. With the steam’s temperature at boiling point, the only remaining source of heat energy to the turbine is the latent heat energy of phase B. This heat energy will lead to an undesirable circumstance for the operation of our power hungry turbine as we will see next week. ________________________________________ |
Superheating, Part 2
Sunday, August 25th, 2013|
Last time we added a piece of equipment called a superheater, positioned between the boiler and steam turbine, to our basic electric utility power plant steam and water cycle. Its addition enables a greater and more consistent supply of heat energy to the steam which powers the turbine. How much more? Let’s look at Figure 1 to get an idea. Figure 1
You may have noticed that our illustration lacks numerical representation. That’s because power plants are designed differently, depending on fuels used and power output required. So unless we’re talking about a particular power plant, number values would be impractical. For example, I could specify a boiling point of 596°F at 1,500 pounds per square inch (PSI), and a superheater outlet temperature of 1,050°F at 1,200PSI, and I could make note of esoteric things like enthalpy (British Thermal Units per pound mass) values on the Heat Energy axis. But to facilitate our discussion we’ll keep things simple and focus on the general process. Figure 1 shows in phase D the additional heat energy being added to the steam, thanks to the superheater. This is significantly more than had been added by the boiler alone, as represented by phase C. The turbine consumes heat energy added in phases C and D and converts it into mechanical energy to drive the generator, resulting in electrical energy being provided to consumers in the most energy efficient way possible. But increasing power output and efficiency isn’t the superheater’s only job. The heat it adds during phase D ensures the turbine’s safe operation when it’s cranking at full capacity, as represented by the superheated steam zones of phases C and D. Next week we’ll discover how the superheater prevents a destructive process known as condensing from occurring inside the turbine. ________________________________________ |
Superheating, Part I
Monday, August 19th, 2013|
Last time we learned that our power plant boiler as presently designed doesn’t do a good job of producing ample amounts of superheated steam, the kind of steam that turbines need to spin and power generators. During the process of superheating the more heat energy that’s added to the steam in our boiler, the higher its temperature becomes. However, our boiler can only produce a limited amount of superheated steam as it stands now. So how do we get more heat energy into the superheated steam? Take a look at the illustration below for the solution to the problem. You’ll note a prominent new addition to our illustration. It’s called a superheater. The superheater performs the function of raising the temperature of the steam produced in our boiler to the incredibly high temperatures required to run steam turbines and electrical generators down the line, as explained in my blog on steam turbines. The superheater adds more heat energy to the steam than the boiler can alone. In fact, the amount of heat energy in the superheated steam produced with our new design is proportional to the amount of electrical energy that power plant generators produce. Its addition to our setup will result in more energy supplied to the turbine, which in turn spins the generator. The result is more electricity for consumers to use and a more efficiently operating power plant. But inefficiency isn’t the only problem addressed by the superheater. We’ll see what else it can do next week. ________________________________________ |
Heat Energy Within the Power Plant— Water and Steam Cycle, Part 2
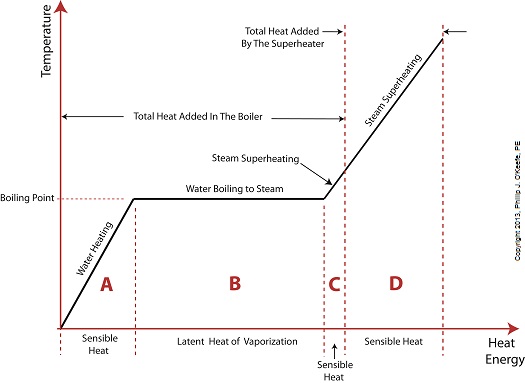
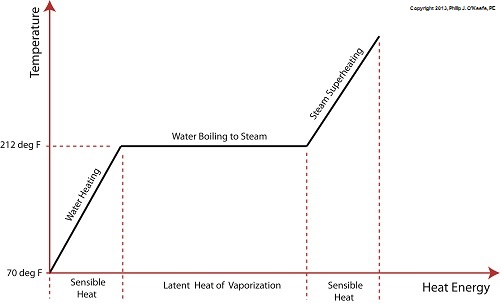
Wednesday, August 14th, 2013|
Last time we learned that electric utility power plants must have water treatment systems in place to remove contaminants from incoming feed water before it can be used. This clarified water is then fed to a boiler by the boiler feed pump as shown below. As it stands this setup will work to provide electricity, however in this state it’s both inefficient and wasteful. We’ll see why in a minute. Boilers, as their name implies, do a great job of heating water to boiling point to produce steam. They do this by adding the heat energy produced by burning fuel, such as coal, to water, then steam. We learned in earlier blogs in this series that the energy used to heat water to boiling point temperature is known as sensible heat, whereas the heat energy used to produce steam is known as latent heat. The key distinction between these two phases is that during sensible heating there is a rise in temperature, during latent heating there is not. For a review on this, see this blog article. When water starts to heat inside the boiler, sensible heat energy is said to be added. This is represented by phase A of the graph below. During A, heat energy will raise the temperature of the water to boiling point. As the water continues to boil in phase B, water is transforming into steam. During this phase latent heat energy is said to be added, and the temperature will remain at boiling point. In phase C something new takes place. The temperature rises beyond boiling point and only steam is present. This is known as superheated steam. For example, if the boiler pressure is at 1,500 pounds per square inch, steam becomes superheated at temperatures greater than 600°F. Unfortunately, boilers alone do a poor job of superheating steam, that is, continuing to raise the temperature of the steam present in phase C. This is evident by the fact that phase C is quite small in comparison to phases A and B before it. This inefficiency in producing ample amounts of superheated steam results in a small amount of useful energy being provided to the turbine down the line, which is bad, because steam turbines require exclusively superheated steam to run the generator. Next time we’ll see how to provide our steam turbine with more of what it needs to run the generator, more superheated steam. ___________________________________________
|
Heat Energy Within the Power Plant—The Power Behind the Turbines
Monday, July 29th, 2013|
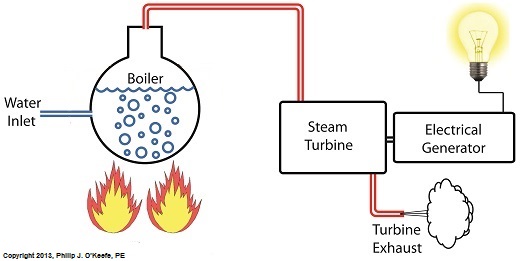
Last time we discovered that the boiling point of water varies. It’s dependent upon the amount of pressure exerted on its surface, which varies due to a variety of reasons, including where it is in relation to sea level. Before we see what happens under higher than atmospheric pressures, such as exist in an electric utility power plant boiler, let’s cover some basics. In the power plant, water is heated in a boiler specifically to produce steam, unlike our tea kettle where the primary purpose is to produce hot water. The steam produced is used to spin turbine generators, which in turn generate electricity, as I explained in a previous blog on steam turbines. Unlike a tea kettle, which is open to the atmosphere on your kitchen stove, the boiler in a power plant is an enclosed, reinforced steel vessel. See illustration below. The reinforced steel boiler vessel is designed to withstand great internal pressure as temperatures rise within. In addition to providing a safety feature, the enclosed space provides a sheltered environment for collecting steam so it can later be put to use spinning power generating turbines down the line. In other words, surface water inside the boiler is closed off from the surrounding atmosphere, allowing its internal pressure to build for our specific purposes. As heat energy is added to water within the boiler, the water boils and steam bubbles break out from its surface, filling the empty space above the surface with pressurized steam. This steam will try to expand here, but it can’t, because it’s being constrained by the reinforced steel vessel within which it is enclosed. Instead, steam pressure builds up on the surface of the water inside the boiler until it is high enough to be released through an attached pipe which is connected to a nearby turbine. We’ll talk more about this pent-up energy and how it is put to use within the power plant in next week’s blog. ___________________________________________
|
Forms of Heat Energy – Boiling Water and Atmospheric Pressure
Sunday, July 21st, 2013|
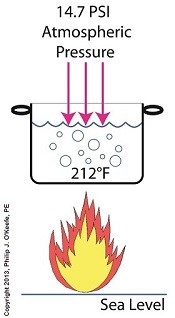
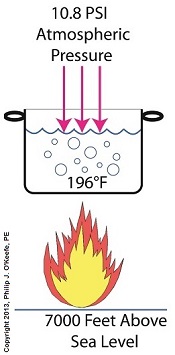
If you’ve ever baked from a pre-packaged cake or cookie mix, you’ve probably noticed the warning that baking times will vary. That’s because the elevation of the area in which you’re doing the baking makes a difference in the baking time required. Living in New Orleans? Then you’re at or below sea level. In Colorado? Then you’re above sea level. Your cake will be in the oven more or less time at the prescribed temp, depending on your location. Last time we learned how the heat energy absorbed by water determines whether it exists in one of the three states of matter, gas, liquid, or solid. We also learned that at the atmospheric pressure present at sea level, which is about 14.7 pounds per square inch (PSI), the boiling point of water is 212°F. At sea level there are 14.7 pounds of air pressure bearing down on every square inch of water surface. Again, I said sea level for a reason. The boiling point of water, just like cake batter baking times, is dependent upon the amount of pressure that’s being exerted on its surface from the surrounding atmosphere. When heat energy is absorbed, it causes the water or cake batter molecules to move around. In fact, the temperature measured is a reflection of this molecular movement. As more heat energy is absorbed, the molecules move more and more rapidly, causing temperature to increase. When the water temperature in our tea kettle reaches its boiling point of 212°F at sea level, the steam molecules in the bubbles that form have enough energy to overcome the atmospheric pressure on the surface of the water. They become airborne and escape in the form of steam. If we’re up in the Rockies at say an altitude of 7000 feet above sea level, the atmospheric pressure is only about 10.8 PSI. There’s just less air up there. That means there’s less air pressure resting upon the surface of the water, so it’s far easier for steam molecules to form into bubbles and leave the surface. As a result the boiling point is much lower in the Rockies than it is at sea level, 196°F versus 212°F. So what if the water was boiling in an environment that had even higher pressures exerted upon it than just atmospheric? We’ll see how to put this pent-up energy to good use next week, when we begin our discussion on how steam is used within electric utility power plants.
___________________________________________
|