|
Last time we learned that the addition of a superheater to the electric utility power plant steam cycle provides a ready supply of high temperature steam, laden with heat energy, to the turbine, which in turn powers the generator. But this isn’t its only job. One of the superheater’s most important functions is to regulate the ongoing process of desuperheating that takes place as the turbine consumes heat energy. To understand this, let’s see what takes place if the superheater were to be removed from its position between the boiler and turbine. Figure 1
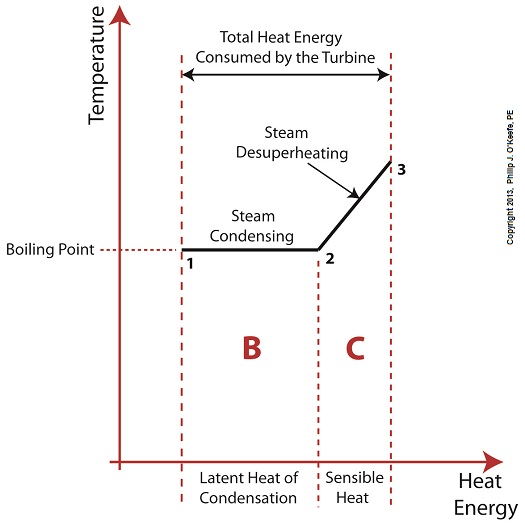
Without the superheater, the only available remaining source of sensible heat energy to the turbine would come from the meager amount present in phase C steam as shown in Figure 1. If you’ll recall from a past blog, the sensible heat energy contained in superheated steam is the best source of energy for a steam turbine, because it’s able to keep it operating most efficiently. As the turbine consumes the heat energy in phase C, starting at point 3 and continuing to point 2, the steam it’s consuming is in the process of desuperheating, as evidenced by the downward slope between the two points. Desuperheating is an engineering term which means that as sensible heat energy is removed from the steam due to its use by the turbine, there will be a resulting drop in steam temperature. And if this process were to continue without the compensatory function provided by the addition of a superheater to the steam cycle, the steam’s temperature would eventually return to mere boiling point, at point 2. This is an undesirable thing. With the steam’s temperature at boiling point, the only remaining source of heat energy to the turbine is the latent heat energy of phase B. This heat energy will lead to an undesirable circumstance for the operation of our power hungry turbine as we will see next week. ________________________________________ |
Posts Tagged ‘power plant engineering’
Desuperheating in the Steam Turbine
Monday, September 2nd, 2013Forms of Heat Energy – Sensible
Sunday, July 7th, 2013| In our house the whistle of a tea kettle is heard throughout the day, no matter the temp outside. So what produces that familiar high pitched sound?
When a tea kettle filled with room temperature water, say about 70°F, is heating on the stove top, the heat energy from the burner flame will transfer to the water in the kettle and its temperature will steadily rise. This heat energy that is absorbed by the water before it begins to boil is known as sensible heat in thermodynamics. To read more about thermodynamics, click on this hyperlink to one of my previous blog articles on the topic. So, why is it called sensible heat? It’s so named because it seems to make sense. The term was first used in the early 19th Century by some of the first engineers who were working on the development of boilers and steam engines to power factories and railways. Simply stated, it’s sensible to assume that the more heat you add to the water in the kettle, the more its temperature will rise. So how high will the temperature rise? Is there a point when it will cease to rise? Good questions. We’ll answer them next week, along with a discussion on another form of heat energy known as the latent heat of vaporization. ___________________________________________
|