|
Last time we discussed how Galileo proved Aristotle’s theory regarding the physics of falling objects to be wrong, although his experiment, which took place on the infamous Leaning Tower of Pisa, did not actually prove his own theory to be correct. So why didn’t Galileo go the extra mile and prove his theory? Because he couldn’t. Galileo, of course, resided on Earth, which was also the arena in which his experiment took place. As such, both he and his experiment were subject to the physical constraints presented by the Earth lab, the single most influential factor being the impact of the planet’s atmosphere upon his falling objects. Put another way, contrary to popular belief at the time, air is not an empty, innocuous space devoid of physical properties. It’s actually a gaseous soup of molecules. Nitrogen, oxygen, carbon, hydrogen, and other elements are in the mix, and they all have mass, that is, weight within a gravitational field. As Galileo’s balls fell, they continuously bumped against these molecules, which slowed their descent. This air friction will be discussed later in our blog series. But in order to prove Galileo’s theory correct beyond a shadow of a doubt, the testing arena would need to be one free from the interference of atmosphere. The Moon fits this criterion and provided the perfect environment to prove, once and for all, that Galileo’s theory was correct. So when astronauts Scott and Irwin simultaneously dropped a hammer and feather to the Moon’s surface, both objects hit at precisely the same moment. Watch this captured live footage of the event to see for yourself: One thing you may have noticed while watching the astronauts’ experiment is that the hammer fell more slowly than it would have on Earth. This has nothing to do with the absence of atmosphere on the Moon, but it has everything to do with gravity. We’ll discuss gravity’s influence in detail next time. _______________________________________
|
Posts Tagged ‘atmosphere’
Proving Galileo’s Theory On Falling Objects
Thursday, September 11th, 2014Forms of Heat Energy – Boiling Water and Atmospheric Pressure
Sunday, July 21st, 2013|
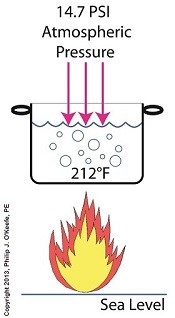
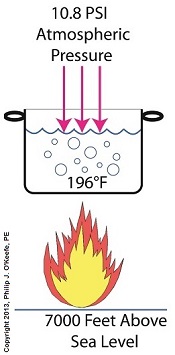
If you’ve ever baked from a pre-packaged cake or cookie mix, you’ve probably noticed the warning that baking times will vary. That’s because the elevation of the area in which you’re doing the baking makes a difference in the baking time required. Living in New Orleans? Then you’re at or below sea level. In Colorado? Then you’re above sea level. Your cake will be in the oven more or less time at the prescribed temp, depending on your location. Last time we learned how the heat energy absorbed by water determines whether it exists in one of the three states of matter, gas, liquid, or solid. We also learned that at the atmospheric pressure present at sea level, which is about 14.7 pounds per square inch (PSI), the boiling point of water is 212°F. At sea level there are 14.7 pounds of air pressure bearing down on every square inch of water surface. Again, I said sea level for a reason. The boiling point of water, just like cake batter baking times, is dependent upon the amount of pressure that’s being exerted on its surface from the surrounding atmosphere. When heat energy is absorbed, it causes the water or cake batter molecules to move around. In fact, the temperature measured is a reflection of this molecular movement. As more heat energy is absorbed, the molecules move more and more rapidly, causing temperature to increase. When the water temperature in our tea kettle reaches its boiling point of 212°F at sea level, the steam molecules in the bubbles that form have enough energy to overcome the atmospheric pressure on the surface of the water. They become airborne and escape in the form of steam. If we’re up in the Rockies at say an altitude of 7000 feet above sea level, the atmospheric pressure is only about 10.8 PSI. There’s just less air up there. That means there’s less air pressure resting upon the surface of the water, so it’s far easier for steam molecules to form into bubbles and leave the surface. As a result the boiling point is much lower in the Rockies than it is at sea level, 196°F versus 212°F. So what if the water was boiling in an environment that had even higher pressures exerted upon it than just atmospheric? We’ll see how to put this pent-up energy to good use next week, when we begin our discussion on how steam is used within electric utility power plants.
___________________________________________
|