| If you’re a fan of the new hit HBO series, Boardwalk Empire, then you know a lot about the effects of Prohibition. But did you know that Prohibition is responsible for the creation of mixed drinks? Until then, people drank their liquor straight. Then along came Prohibition, mob rule, and the desire to keep the booze, including some with questionable origins, flowing. This booze didn’t taste so good, and the addition of a sugary beverage to it, that is, diluting it with soda or juice, made it a lot easier to go down. By the time Prohibition was repealed in 1933, the mixed drink had taken a firm foothold in American society.
Most adults are aware of the fact that liquor, in excess, is toxic to the body. Too much of it, and the liver, which acts as a filtration device, itself becomes toxic. When that happens, poor health will follow. The same principle applies to air within a building. If it becomes thick with toxic fumes or potentially flammable vapors, indoor air quality will suffer. But if you infuse fresh air into the environment, the toxic load is diluted, making the environment habitable and safe. This addition of fresh air is called, appropriately enough, dilution ventilation. Now, the easiest way to create a dilution ventilation system is to open a window. Trouble is there often isn’t enough natural airflow to do much good. But if you step up the effort by introducing a mechanical ventilation system, complete with blowers and ductwork, the need to crack open a window becomes obsolete. By exchanging bad air for good and introducing a continual flow of fresh air, toxicity is diluted and its effects are minimized, much like the bathtub gin of Prohibition was improved by the addition of soda. The chance of fire or explosion is reduced as well. There are however limits to what dilution ventilation can accomplish. If contaminants are highly toxic or extremely flammable, then this type of ventilation system is not going to do much good. This is a situation where extremely high air flows would be required, and this is often impractical both from a cost and comfort standpoint. Imagine having to work inside a wind tunnel? In situations like these a local exhaust ventilation system is better suited to do the job, and we’ll see how those work next week. _____________________________________________ |
Posts Tagged ‘explosion’
Industrial Ventilation – Dilution Ventilation
Sunday, April 3rd, 2011Pressurized Containers – Industrial Overpressure Devices
Sunday, October 17th, 2010| Perhaps you went out on a drive to enjoy a nice summer day. As you ventured into uncharted territory, you might have ended up in an industrial area. There, you noticed factories, chemical plants, and oil refinery complexes, each surrounded by a huge system of pipes and tanks. You might have considered it to be an eyesore, but if you’re an artist and engineer like I am, you might look at it as a form of art, composed of interesting shapes, colors, and patterns. No matter how you look at it, you can bet that there are at least a few pressurized containers in there.
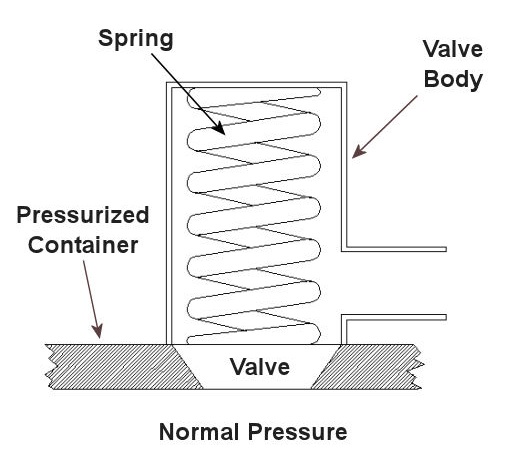
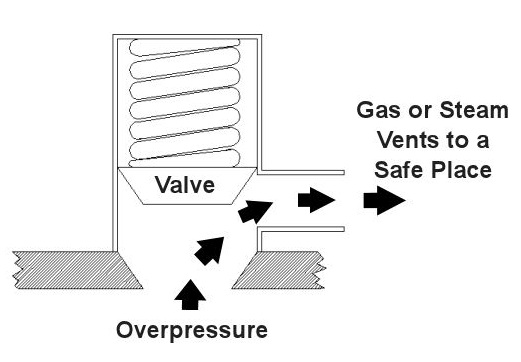
Last time we saw how something as seemingly harmless as a home water heater could become a dangerous missile if the pressure inside builds to the point where the tank ruptures. You can imagine what kind of explosive forces, steam, and chemicals would be unleashed into the surroundings if an industrial sized pressurized container failed due to overpressure. Let’s explore some other types of overpressure devices that are commonly used in industrial settings. One type of overpressure device is a safety valve. They are similar to a water heater relief valve, but they are generally used to relieve overpressure of gases and steam. How do they work? Basically, a safety valve is attached to the top of a pressurized container as shown in the cut away view in Figure 1 below. Figure 1 – A Basic Safety Valve In The Closed Position A powerful spring in the valve body is designed to force down on the valve and keep it closed if there is normal pressure inside the container. Once the pressure begins to rise to an unsafe level, it pushes up against the valve and overcomes the force of the spring. The valve opens, as shown in Figure 2 below, and the contents of the pressurized container are safely vented out to an area that is normally unoccupied by people. In case you’re wondering, safety valves are commonly used on pressurized storage tanks and boilers. Figure 2 – A Basic Safety Valve In The Open Position Another way to address the overpressure scenario is to employ a rupture disc. This is in fact a purposely constructed weak spot. It is intentionally built into a pressurized container and is designed so that it will fail when pressure starts to rise. In fact, this disc is designed to fail at a pressure point just below the pressure at which the container itself would fail. The disc is usually located within a vent pipe, which is in turn connected to the container. Should the disc rupture in an overpressure situation, the contents of the pressurized container will safely flow out of the vent pipe to a place normally unoccupied by people. The advantage of using a rupture disc is that they are made to safely release huge quantities of pressurized substances very quickly. The disadvantage in their usage is that they’re a one-time fix. That is, unlike relief or safety valves which may perform their function a multitude of times, a rupture disc is destroyed once it does its job. They are generally used in industrial settings where potential hazards are greater than at home, so once the rupture disc blows, the complete system generally undergoes a shut down so that the disc an be replaced before the pressurized container can be used again. Another option to pressure containment is the use of a fusible plug, usually constructed of a metal that will melt if the temperature within a pressurized container rises above a certain level. The metal plug melts, and excess pressure is vented through the aperture formed into a safe location. These are often used on locomotive boilers and compressed gas cylinders. Like rupture discs, fusible plugs are a one-time fix and must be replaced once they have done their job. Yet another option to pressure containment is to use a temperature limiting control. This category includes devices that monitor temperature and pressure within a pressurized container. If a dangerous situation should develop, the control system reacts, effectively reducing the pressure to prevent failure of the vessel. Automatic combustion control systems for boilers in electric utility power plants use temperature and pressure sensors to keep pressures within safe limits by regulating fuel and air input to the boiler. Next time we’ll cover the American Society of Mechanical Engineers (ASME) Boiler and Pressure Vessel Code (BPVC), which establishes rules governing the design, fabrication, testing, inspection, and repair of boilers and other pressurized containers. _____________________________________________ |
Pressurized Containers
Sunday, September 19th, 2010| My dear daughter is at times forgetful? On one occasion in particular she forgot that she had placed a can of pop in the freezer to speed-chill it. Later that evening when my wife went to get something out of the freezer she was not too pleasantly surprised to find that a black, semi-solid mess had covered most of the freezer’s interior. You got it. The pop can exploded, shooting its pressurized contents all over the place.
You may not have thought much about it before, but pressurized containers are all around us, from pop to aerosol cans, car tires, water heaters, and liquid petroleum gas tanks. Pressurized containers are even more obvious in industrial settings such as oil refineries, power plants, and factories. As its name would imply, pressurized containers, or pressure vessels, are under a lot of pressure, as such, they are no stronger than their weakest point. Whether that point be its sides, as in the case of my daughter’s ruptured pop can, or its ends, weld joints, rivets, or any of the other components that are included in the vessel’s construction. As with anything, the integrity of something is only as good as its weakest part. In an industrial setting, this fact, depending on the vessel’s contents and the severity of the failure, can prove deadly. So what causes pressure in a vessel to get too high? Many factors could be at play, from a safety valve which fails to do its job of relieving excess pressure, to absence of this feature entirely. Many of us learned in grade school science class that when a substance heats up, its molecules vibrate, causing its atoms to want to distance themselves from each other. If this vibrating is taking place within a closed vessel, heated atoms will be prevented from carrying out their desired separation. The result is pressure increases, and along with it the propensity for the weakest points to fail. Whether it’s a slow ooze, high spirited fizz, or outright explosion, the end result is generally the same – it’s a big mess. The behavior of gases in pressurized vessels exposed to heat can be summed up by the Boyle-Charles Law, named after the 17th Century scientist, Robert Boyle, and a 19th Century scientist named Jacques Charles. Both were pioneers in their study of how gases behave under various conditions. The science behind their observations can be summarized into a neat little formula, known as the Boyle-Charles Law for sealed pressure vessels. It looks like this: P1÷T1 = P2÷T2 where P is absolute pressure and T is absolute temperature. What is absolute pressure? It’s the pressure that’s measured when you add the pressure of the air that we live in, our atmosphere, which has been measured to be 14.7 PSI, or pounds per square inch, to whatever pressure you are reading on a pressure gauge. This ultimate pressure is measured at pounds per square inch absolute, or PSIA. Now what is meant by absolute temperature is calculated a little differently. It basically means that you add 460 degrees to the temperature reading on a thermometer. This 460 degrees acts as a kind of fudge factor to keep equations that concern themselves with temperature at or below 0°F to work. The result is said to be in degrees Rankine, and it is denoted by °R. So why do we need this fudge factor? Here’s an example. In the equation above, if temperature is 0°F, then you would be dividing the pressure by zero in the equation above. That’s a no-no in mathematics. Try dividing any number by zero on your calculator and see what I mean. Okay, for those of you who are not mathematically inclined that may not have been very clear. Let’s try a different approach. Probably the best way to show you how the Boyle-Charles Law works is by the following example. Suppose you have a sealed container filled with pressurized gas. The gas is at a temperature of 70°F, and a pressure gauge on the vessel reads 100 PSI. So this condition of pressure and temperature goes into the Boyle-Charles Law equation as P1 = (100 PSI + 14.7 PSI) = 114.7 PSIA and T1 = (70°F + 460) = 530°R. Now let’s introduce a complicating factor. Suppose you leave the container in your car on a hot day. The gas in the container increases in temperature to 150°F. Okay, so this temperature would go into the Boyle-Charles Law equation as T2 = (150°F + 460) = 610°R. Now, suppose the pressure gauge was damaged and the needle fell off. What would the gas pressure in the container be at this temperature? Let’s use the Boyle-Charles Law equation and a little algebra to find out: P1÷T1 = P2÷T2 114.7 PSIA ÷ 530°R = P2÷ 610°R P2 = (114.7 PSIA ÷ 530°R) × 610°R P2 = 132 PSIA = 117.3 PSI What this means is that if the weakest link in a container was made to withstand no more than 110 PSI of pressure, and the pressure inside the vessel in our case has risen to 117.3 PSI, the integrity of our container will be compromised. We’ll return to our vehicle and find a big mess. We’ll continue our discussion of pressure vessels, leaks, and how they can be prevented in the future. _____________________________________________ |