Posts Tagged ‘lab testing’
Sunday, June 22nd, 2014
|
Last time we learned that gear trains are torque converters, and we developed a torque ratio equation which mathematically ties the two gears in a gear train together. That equation is:
T1 ÷ T2 = D1 ÷ D2
Engineers typically use this equation knowing only the value for T2, the torque required to properly drive a piece of machinery. That knowledge is acquired through trial testing during the developmental phase of manufacturing.
Once T2 is known, a stock motor is selected from a catalog with a torque value T1 which closely approximates that of the required torque, T2. Then calculations are performed and lab tests are run to determine the driving and driven gear sizes, D1 and D2 which will enable the gear train to convert T1 into the required value of T2. This series of operations are often a time consuming and complex process.
To simplify things for the purpose of our example, we’ll say we’ve been provided with all values required for our equation, except one, the value of T2. In other words, we’ll be doing things in a somewhat reverse order, because our objective is simply to see how a gear train converts a known torque T1 into a higher torque T2.
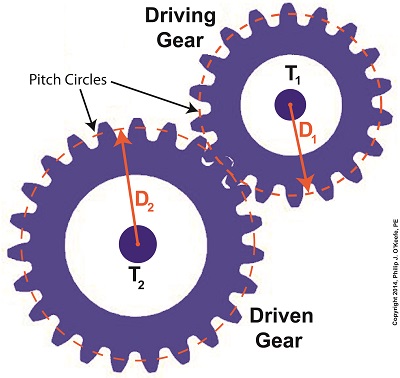
We’ll begin by considering the gear train illustration above. For our purposes it’s situated between an electric motor and the lathe it’s powering. The motor exerts a torque of 200 inch pounds upon the driving gear shaft of the lathe, a torque value that’s typical for a mid sized motor of about 5 horsepower. As-is, this motor is unable to properly drive the lathe, which is being used to cut steel bars. We know this because lab testing has shown that the lathe requires at least 275 inch pounds of torque in order to operate properly.
Will the gears on our gear train be able to provide the required torque? We’ll find out next time when we insert values into our equation and run calculations.
_______________________________________
|
Tags: calculations, driven gear, driving gear, driving gear shaft, electric motor, engine lathe, engineering, engineering expert witness, forensic engineer, gear expert, gear train, gears, horsepower, lab testing, lathe, machinery expert, motor torque, shaft, torque, torque calculations, torque converter
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability | Comments Off on The Methodology Behind Gear Train Torque Conversions
Sunday, February 24th, 2013
| Done any remodeling lately? If you have, you’ve been faced with countless choices regarding design and materials. Even a relatively simple decision such as putting in hardwood flooring requires many considerations. What type of wood? What grade? How about the stain? Should it be factory stained and sealed, or should the flooring be installed by single board, then stained and sealed in place? Ultimately, your decision is based on your requirements with regards to cost, durability, and personal style.
Now imagine the decision making process that is required to produce a medical device. We’ve been discussing this complex process during our series on medical device design utilizing the systems engineering approach, a systemized approach to product development, design, and manufacture that is used within many manufacturing arenas. Its objective is to relate the requirements for manufacture, regulatory compliance, sale, use, and maintenance of the product to specific design criteria for functionality, durability, and safety. By doing so, the systems engineering approach ensures that the product meets or exceeds all requirements.
Last time we wrapped up our discussion on the Development stage of systems engineering by discussing field testing of medical devices assembled during Preproduction. Problems encountered during this phase result in a comprehensive review of the device design and instructions. When all issues have been resolved, things move on to the manufacturing phase and full commercial production.
During the Production stage, engineers make continual assessments of the manufacturing process and ongoing adjustments are made to the device design and manufacturing protocol as necessary, this due primarily to changing stakeholder requirements regarding cost reduction. In the competitive marketplace, cost reduction is a never-ending quest to maintain profitability in view of changing economic and market conditions, and this must be done without compromising the quality, safety, and effectiveness of the device.
For example, suppose a medical diagnostic imaging machine was designed to be fitted with a machined metal gear in one of its mechanisms. The manufacturer specifies that a $10 decrease must be made in production costs so it can continue to be sold at an acceptable profit margin. After reviewing the design, engineers discover that substitution of a molded plastic gear would reduce manufacturing cost per machine by $12. This is a common scenario, as plastic parts are often substituted for metal to save on cost.
Plastic versus metal? How can that be an acceptable swap? In many cases, it can be. Mechanical stressors are analyzed, and if the plastic gears meet durability requirements as well as their metal counterpart, they are substituted. During the Preproduction phase these plastic gears are used in both lab and field testing, where they are put through the rigors of real world use. If they perform acceptably, they are made a permanent part of the device’s design and used in commercial production.
Next time we’ll continue our look at the Production stage to discover another way that systems engineering can facilitate cost reduction to meet stakeholder requirements.
___________________________________________
|
Tags: commercial production, cost reduction, design criteria, Development Stage, diagnostic imaging machine, durability, effectiveness, engineering expert witness, field testing, forensic engineer, functionality, gear, lab testing, machined gear, manufacturability, manufacturing costs, mechanical stressors, medical device design, metal, plastic, product development, Production Stage, quality, regulatory compliance, safety, systems engineering
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability | Comments Off on Systems Engineering In Medical Device Design – Production, Part 1
Monday, February 11th, 2013
| Last time we began our discussion on Preproduction, the final aspect of the Development stage of our systems engineering approach to medical device design. This is the point at which a small amount of devices are put into actual production, then evaluated for full production possibility. It is also the final juncture at which problems will be evaluated and corrected before full commercial production can begin.
Once the medical devices produced during Preproduction are assembled, they’re subjected to rigorous testing in both a laboratory and the field. This testing is necessary to see if stakeholder requirements are satisfied. At this stage devices constructed en masse on the factory assembly line are compared to prototypes built by hand by design engineers earlier in the Development stage.
During Preproduction laboratory test data is gathered and analyzed by engineers to assess how the device will hold up during actual use. Real-life conditions are simulated in the lab environment to facilitate this process. For example, lab testing of a Preproduction kidney dialysis machine can determine whether its blood pump flow rate falls within acceptable range during hundreds of hours of operation. Other factors, such as durability of materials are evaluated during lab testing. In the case of the dialysis machine, there is a component called a dialyzer that filters toxic waste from blood. Over the duration of the lab test, the material used in the dialyzer filter membranes would be inspected and evaluated for durability.
Next week we’ll conclude our discussion on Preproduction to see what happens when testing is moved outside the lab environment into the field.
___________________________________________
|
Tags: design engineer, design revision, Development Stage, durability of materials, engineering expert witness, factory assembly line, field testing, filter, filter membrane, flow rate, forensic engineer, lab testing, machine, maintenance instructions, manufacturing, medical device design, operating instructions, preproduction medical device, Production Stage, project stakeholder, pump, systems engineering in medical device design, test data, Utilization Stage
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, Product Liability | Comments Off on Systems Engineering In Medical Device Design – Preproduction, Part 2






