|
Did you know that from the early days of the Industrial Revolution until well into the 20th Century it was common practice for all aspects of a product to be built entirely under one roof? For example, a wheelchair manufacturer in the 1890s would buy the various raw materials needed to construct component parts, everything from bars of steel and wooden boards to rattan stalks and gum rubber, then produce every part of the wheelchair in one facility. Items as diverse as chair frames, footrests, wicker seat cushions, springs, wheel rims and spokes, and tires would all be constructed from the raw materials purchased, then assembled into the finished product. Doesn’t sound like an efficient process to you? Henry Ford didn’t think so either. In fact, he is credited with pioneering mass production in manufacturing when he observed during the production process of his line of automobiles that inefficiencies abounded. Inefficiencies in manufacturing are common, as they are in everyday life. Last time we saw how robots, i.e., the introduction of industrial automation, can be used during the Production stage of our systems engineering approach to medical device design to increase efficiency and reduce manufacturing costs. Today we’ll take a look at another inefficient practice, along with its solution. Returning to our wheelchair manufacturer, the problems associated with manufacturing and assembling all aspects of a product are many. At the top of the list is the substantial cash outlay that’s required to buy and maintain a huge factory complex and all the specialized equipment required to make each and every part. In addition, there’s the ongoing expense of employing and training employees needed to fabricate each component. In other words, the wheelchair factory has a lot of fixed overhead expense to carry, and the more overhead there is, the more expensive the end product. Expenses such as these are almost always passed on to the buyer. The solution? Outsourcing. That is, using outside manufacturers to produce many, perhaps even all, of the component parts. Then our wheelchair manufacturer would simply assemble the purchased parts into the finished product, resulting in lower manufacturing costs and higher profits. The benefits of outsourcing were widely recognized in the decades following World War II, when the post-war economy was booming and demand for consumer goods increased dramatically. That ends our look at the Production stage. Next time we’ll move on to the Utilization stage to see how the systems engineering approach is put into play once the medical device has been introduced into the marketplace. ___________________________________________ |
Posts Tagged ‘medical device manufacturing’
Systems Engineering In Medical Device Design – Production, Part 4
Sunday, March 17th, 2013Systems Engineering In Medical Device Design – Production, Part 3
Sunday, March 10th, 2013| When I was a kid I had a toy robot that captured my attention like no other toy. I thought it was so cool to have something animated that looked both humanoid and machine-like at the same time. It couldn’t do much, just walk in a stiff, jerky way and move its arms up and down, but that was enough to keep me fascinated.
Today’s generation of robots do not often take on the humanoid form, but they’re capable of so much more. Robots on assembly lines perform a variety of tasks like welding and placing electronic components on circuit boards, and they do it much more quickly and accurately than any human could, so they’re often employed in manufacturing. We’ve been discussing the Production stage of the systems engineering approach to medical device design. We learned that within the manufacturing process there are often opportunities for cost reduction, and today we’ll see how robots can be used to reach those goals. Last week we presented a sample scenario involving the manufacture of a percussion therapy device. In their quest to reduce manufacturing costs, engineers identified bottlenecks along the assembly line which led to idle worker time and the inability to keep up with orders. In addition to these production woes, it was discovered that the tedious, repetitive manual labor that occurred at each bottleneck created opportunities for assembly mistakes. As many as 30 devices per day were being rejected by quality control inspectors due to issues such as faulty wiring and improper parts usage. This led to expensive rework to correct mistakes. After further evaluation, design engineers determine that bottlenecks can be eliminated by installing automated assembly equipment in the three distinct assembly stages represented on the line, those involving wiring harnesses, printed circuit boards, and the motor drive mechanism. The potential for human error is high during many facets of manufacturing, and this can be minimized or eliminated through the use of robots, that is to say, mechanized equipment capable of automatically performing a complex series of specific tasks. These robots never tire of performing tedious, repetitive work, and their efficiency is unparalleled. Their introduction at key junctures on the assembly line has benefits across the manufacturing process, enabling workers to keep continuously busy and reducing the incidence of human error. The introduction of robotics is known as industrial automation. Robots efficiently increase manufacturing speed, and along with it profits, so their introduction more than compensates for the investment costs associated with purchasing them. Next time we’ll continue our look at the Production stage to discover another way that systems engineering can simplifying the assembly process, by eliminating some functions altogether. ___________________________________________ |
Systems Engineering In Medical Device Design – Preproduction, Part I
Monday, February 4th, 2013| If you’ve been following along with our blog discussion on the systems engineering approach to medical device design, you should by now be convinced that instructions are important. In fact, the meticulous instructions produced during the manufacturing, operating, and maintenance phases of the Development stage are also crucial to later stages, that of Production and Utilization. Let’s finish up our discussion on the Development stage by taking a look at its final aspect, Preproduction.
The Preproduction aspect is instrumental to nipping potential problems in the bud before the medical devices go into actual production. In the initial Preproduction stages, systems engineers coordinate with the manufacturing and purchasing departments within the company as well as outside suppliers. The goal is to acquire all parts and equipment necessary to build a limited number of medical devices on the assembly line. Subjects such as preference in molded plastic components, motors, gears, pumps, springs, electronic components, circuit boards, wire, and tubing are discussed and agreed upon. Vendors are assessed with regard to their ability to produce parts when they are needed and that meet design specifications, satisfy quality requirements, and have costs that fall within budgetary constraints. The assembly of Preproduction devices provides an opportunity for systems engineers to validate manufacturing and quality control instructions and assess the device design with regard to manufacturability, meaning, the extent to which devices can be manufactured with relative ease, at minimal cost, while maintaining maximum reliability. Devices manufactured during this aspect of the Development stage serve as a test. Are instructions clearly written? Do the device parts fit together as they should? Are parts strong enough to withstand the assembly process? Can the devices be assembled as quickly and easily as expected? If the answer is “no” to any of these questions, then the device design and instructions must be returned to the design engineers and technical writers. Heads come together to rehash things and work out the bugs. Next time we’ll continue with the Preproduction aspect of the Development stage to see how laboratory and field testing enables systems engineers to shake out any more bugs from the medical device design, operating instructions, and maintenance instructions. ___________________________________________ |
FDA Classifications for Medical Devices – General Controls
Sunday, August 15th, 2010|
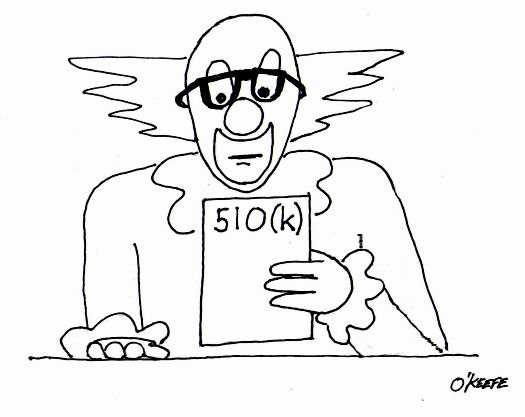
When I was a kid in Chicago back in the 1960s there was a show on television called Bozo’s Circus. Lucky kids were picked from the audience to play a bucket game. There were six buckets in a row, as I remember, each about a foot from the last. The kids had to stand in front of the first bucket to play. By the time the kids got to throw their ball into Bucket No. 6 there was probably ten feet for the ball to travel. So what would happen if the ball didn’t sink into the desired bucket, which would happen more often than not it seemed? Then Ringmaster Ned would direct Cookie the Clown to chase down the rogue balls. So what does this have to do with the FDA and medical devices? Well, in the loosest of terms you may think of the FDA’s classification system as Buckets 1 through 6 and Ringmaster Ned as the regulatory agent of the game. Okay, I’m really stretching on this analogy, but I did want to introduce some levity into the discussion! Last week we discussed the fact that the FDA classifies medical devices into three main categories, Classes I, II and III, Class I devices posing the least risk to patients, Class III the most. Now we’ll see how the FDA regulatory control system functions to oversee the medical devices within each classification. To begin with, you should be aware that regulatory controls are themselves divided into General and Special Control categories. In this article we’ll focus on General Controls. General Controls can apply to medical devices within all three FDA risk classifications. They include requirements for: — Registering of medical device manufacturers, distributors, repackagers, and relabelers with the FDA. This registration basically lets the FDA know they exist as an entity, and it gives the Agency information about who to contact should the need arise. — Listing medical devices with the FDA so the Agency can keep track of what kind of devices are being marketed in the United States. — Manufacturing devices in accordance with FDA Good Manufacturing Practices (GMP). GMP regulations require a quality approach to manufacturing, an approach which is designed to minimize or eliminate instances of contamination, defect, and error which could contribute to harm or kill a patient. GMP regulations address issues like sanitation, quality control, complaint handling, and record keeping. Effective complaint handling and record keeping systems are key in identifying and resolving issues that may pose increased risk to patients. — Labeling devices. The FDA requires that medical device labeling provide explicit directions for use. The labels must also contain appropriate warnings as needed to ensure the safe and effective use of the device. — Submitting a Premarket Notification to the FDA for approval before marketing a device, also referred to as a 510(k) within the industry. This name comes from the section of the federal regulation that deals with it, that is to say, companies must submit 510(k) documents to the FDA to demonstrate that the device they wish to market is comparably safe and effective as other equivalent devices already on the market. A 510(k) can only be submitted if Premarket Approval (PMA) for the device is not required. We’ll talk more about this in a future article on Special Controls. Now, because they pose such a low level of risk, many Class I medical devices are exempt from the requirement for 510(k) submissions altogether, and they may even be exempt from compliance with GMP regulations. In a nutshell, General Controls help the FDA keep track of what products are being sold by whom, and how effective and safe those products are. It also provides guidelines to medical device companies to help ensure their introduction of safe and effective products to the public. So what happens when a device falls outside of the parameters of the General Controls watch? That’s when more stringent guidelines come into play, for it has now entered the realm of Special Controls. _____________________________________________ |