| Ever overdraw on your checking account or max out a credit card? It’s not hard to do if you’re not keeping track of things. How can we manage household expenses without some sort of record keeping?
Away from home, in the business sector, record keeping becomes even more important. In fact, it’s the very thing covered by HACCP Design Principle No. 7. Principle 7: Establish record keeping procedures. – This HACCP principle requires that all food manufacturing plants maintain records to show they implemented a HACCP plan, are following all principles, and the plan is working effectively. Let’s look at an example. In keeping with the directive of HACCP Design Principle 7, the engineering department of a food manufacturing plant must keep records for each design project. The design record for a new cookie forming machine would contain things like engineering calculations to determine strength requirements of machine parts and supports, as well as power requirements for the electric motor that drives the machine. This design record would also contain documentation concerning materials selected to construct the machine, as well as dimensioned mechanical drawings of the machine and its parts. These dimensioned drawings will show all physical dimensions of the machine and its constituent parts. The record would also contain test results and analysis of the results. Lastly, the design record must include a risk analysis of potential hazards that could result. Other activities include identification of CCPs, establishment of critical limits, and other factors in accordance with HACCP Design Principles 1 through 5. In other words, the record must be complete, bearing witness to an effective adherence to HACCP Design Principles 1 through 5. Principle 7 also encompasses guidelines set in place through Design Principle 6, which calls for the establishment of procedures to govern Principles 1 through 5. A complete record would contain the procedures themselves, along with any revisions. It would also contain documentation that the procedures were reviewed and approved by management along the way. Finally, of what use would records be if they were incomplete, disorganized, and outdated? A document control system not only establishes procedures, but assigns responsibilities to personnel within the department for filing design records to make sure that everything is up to snuff. This system would encompass everything, from the creation of engineering documents, to their timely entry into the record keeping system. We have now exhausted our discussion on HACCP Design Principles. We’ll switch to a new topic next time, examining some basic concepts behind the control of industrial equipment and machinery. ____________________________________________
|
Posts Tagged ‘risk analysis’
Food Manufacturing Challenges – HACCP Design Principle No. 5
Saturday, November 12th, 2011| Picture yourself on a highway, it’s dark out, the wind is blowing fiercely, and you’re unable to see that the pavement is accumulating icy patches. You hit one, and your car veers out of control. Luckily you drive one of the new generation of “smart” vehicles. Wheel sensors detect your predicament and immediately initiate a sequence of events to correct the situation and bring your vehicle back into control.
Corrective measures need to be taken in many situations when things go awry, whether they be computer-generated or human-generated corrections, and food manufacturing facilities are not exempt from the process. Let’s look at how this applies within HACCP Design Principle No. 5. Principle 5: Establish corrective actions. – Simply put, when an established critical limit at a designated critical control point (CCP) has been found not to be functioning as intended, thereby exposing consumers to potential food safety issues, design engineers must enact corrective measures to resolve the issue as soon as possible. Let’s return to the example set out in our last article, where we discussed HACCP Design Principle 4. An engineering manager has discovered a problem with the lower critical limits established by her design engineer’s software logic as it concerns a CCP established with regard to cooker temperature. The time and temperature in the logic create a hazardous situation by not taking into account that larger cuts of meat require more cooking time, resulting in them being undercooked. Fortunately, the engineering manager’s diligent and ongoing day-to-day monitoring has alerted her to the error. She immediately provides feedback about it to the design engineer, who makes corrections to the software logic. Problem solved, and all is working well within the food manufacturing plant, right? Yes, but we’re not finished. We have to make sure that a mechanism is set in place to ensure that HACCP Principles 1 through 5 are being followed and that they are actually working to protect consumers from potential food contamination hazards. Next time we’ll take a look at the last of the HACCP Design Principles, No. 6, which concerns itself with maintenance and housekeeping issues. ____________________________________________ |
Food Manufacturing Challenges – HACCP Design Principle No. 1
Sunday, October 16th, 2011| Imagine a doctor not washing his hands in between baby deliveries. Unbelievable but true, this was a widespread practice up until last century when infections, followed by death of newborns, was an all-too common occurrence in hospitals across the United States. It took an observant nurse to put two and two together after watching many physicians go from delivery room to delivery room, mother to mother, without washing their hands. Once hand washing in between deliveries was made mandatory, the incidence of infection and death in newborns plummeted.
Why wasn’t this simple and common sense solution instituted earlier? Was it ignorance, negligence, laziness, or a combination thereof that kept doctors from washing up? Whatever the root cause of this ridiculous oversight, it remains a fact of history. Common sense was finally employed, and babies’ lives saved. The same common sense is at play in the development of the FDA’s Hazard Analysis Critical Control Point (HACCP) policy, which was developed to ensure the safe production of commercial food products. Like the observant nurse who played watchdog to doctors’ poor hygiene practices and became the catalyst for improved hospital procedures set in place and remaining until today, HACCP policy results in a proactive strategy where hazards are identified, assessed, and then control measures developed to prevent, reduce, and eliminate potential hazards. In this article, we’ll begin to explore how engineers design food processing equipment and production lines in accordance with the seven HACCP principles. You will note that here, once again, the execution of common sense can solve many problems. Principle 1: Conduct a hazard analysis. – Those involved in designing food processing equipment and production lines must proactively analyze designs to identify potential food safety hazards. If the hazard analysis reveals contaminants are likely to find their way into food products, then preventive measures are put in place in the form of design revisions. For example, suppose a food processing machine is designed and hazard analysis reveals that food can accumulate in areas where cleaning is difficult or impossible. This accumulation will rot with time, and the bacteria-laden glop can fall onto uncontaminated food passing through production lines. As another example, a piece of metal tooling may have been designed with the intent to form food products into a certain shape, but hazard analysis reveals that the tooling is too fragile and cannot withstand the repeated forces imposed on it by the mass production process. There is a strong likelihood that small metal parts can break off and enter the food on the line. Next time we’ll move on to HACCP Principle 2 and see how design engineers control problems identified during the hazard analysis performed pursuant to Principle 1. ____________________________________________ |
Food Manufacturing Challenges – Avoiding Contamination
Sunday, October 9th, 2011| Perhaps you’ve heard of the non-reciprocal wine and sewage principle. I’m not sure where it originated, but it states that if you add a cup of wine to a barrel of sewage, you still get a barrel of sewage. No brainer, right? Well, consider the flip side. If you add a cup of sewage to a barrel of wine, you also get nothing more than a barrel of sewage. In other words, a small amount of contamination goes a long way.
The premise of this principle also applies within the food manufacturing industry. If you were to add uncontaminated food to garbage, you would just get more garbage, and if you add garbage to food… well, you get it. The term garbage can encompass an endless variety of contaminants, such as broken glass, metal shavings, nuts, bolts, plastic fibers, grease, broken machine parts, errant human body parts, and on and on. Although the FDA does allow for certain levels of natural contaminants, like insect parts and rodent hairs, consumers are never pleased when undesirable elements enter their food supply. It could even be dangerous. When design engineers create food processing machinery and production lines, they must be on the lookout for potential risks of contamination hazards. They must also provide a quick means of mitigation, before contaminants can enter into commercial production. A systematic approach provides the best means of addressing these needs, allowing for a pre-emptive method to ensure food safety. Checklists and procedural policy set in place for these reasons will enable design engineers to identify, assess, and control risks before they turn into hazards. This is where Hazard Analysis Critical Control Point (HACCP) planning comes in. To address these needs, the FDA has set up the HACCP (pronounced, “hass-up”) system, defined as “…a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product.” HACCP is the outgrowth of FDA current Good Manufacturing Practices (cGMP), which are set out in the Code of Federal Regulations pertaining to commercial food processors and manufacturers, Title 21, Part 110, entitled, “current Good Manufacturing Practice in Manufacturing, Packing or Holding Human Food.” Every commercial food processor, regardless of size, must implement a cGMP/HACCP quality assurance program to comply with these regulations. HACCP is a proactive strategy where hazards are identified, assessed, and then control measures developed to prevent, reduce, or eliminate potential hazards. A key element of HACCP involves prevention of food contamination during all phases of manufacturing, and way before the finished food product undergoes quality inspection. This strategy extends into the food manufacturing equipment and production line design process as well. Next time we’ll continue our look at HACCP and how its seven principles are used by design engineers to prevent food product contamination. |
FDA Classifications for Medical Devices
Sunday, August 8th, 2010|
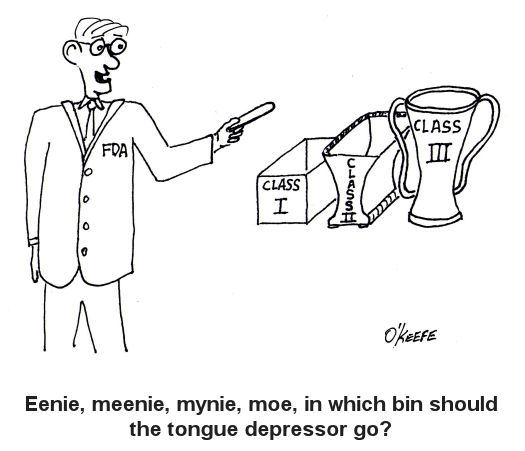
Hardly a week goes by that the FDA, that is the Food and Drug Administration, is not in the news. From the recall of drugs found to be harmful after the fact, to investigations of medical device suppliers and inspections of salmonella contamination at meat processing plants, the FDA is responsible for overseeing and regulating a wide range of products and processes. It’s stated purpose being: The FDA is responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit radiation. http://www.fda.gov/AboutFDA/WhatWeDo/default.htm From its humble beginnings at the beginning of the last century as the Pure Food and Drug Act, it has grown to regulate more than $1 trillion worth of consumer goods, about 25% of that said to be attributable to consumer goods expenditures in the United States. In 1976, the FDA began classifying medical devices, using a three-tiered system to distinguish them according to level of risk to patients. Class I devices present the lowest level of risk and requires the least regulatory control, while Classes II and III represent higher levels of risk. Just to give you some perspective, Class I medical devices include things like tongue depressors, bedpans, arm slings, and hand-held surgical instruments. In the Class II category are things like surgical drapes, blood pressure cuffs, catheters, wheelchairs, heating pads, and x-ray film processing machines. And I’m sure you’ve guessed by now that Class III devices are for the heavy-hitters, including things like defibrillators, heart valves, and implanted cerebral stimulators. So why does the FDA classify medical devices? Practicality is one key reason. It would be highly impractical, if not downright impossible, for the FDA to subject manufacturers of low risk Class I devices, like tongue depressors, to the same scrutiny as manufacturers of Class III devices, such as heart valves, etc. Along with miles of red tape would come a huge financial burden that would effectively raise the price of your common tongue depressor through the roof and, undoubtedly, force the manufacturer out of business. Hand in hand with medical device classifications are regulatory controls imposed by the FDA. We’ll see how they fit into the picture next time. _____________________________________________ |