|
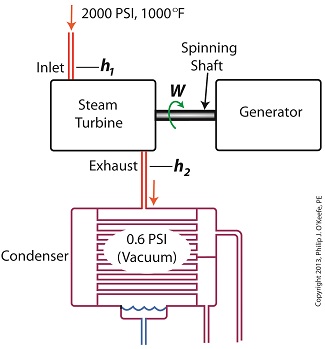
Last time we ran our basic power plant steam turbine without a condenser. In that configuration the steam from the turbine exhaust was simply discharged to the surrounding atmosphere. Today we’ll connect it to a condenser to see how it improves the turbine’s efficiency. As discussed in a previous blog, enthalpy h1 is solely dependent on the pressure and temperature at the turbine inlet. For purposes of today’s discussion, turbine inlet steam pressure and temperature will remain as last time, with values of 2,000 lbs PSI and 1000°F respectively, and calculations today will be based upon those values. So to review, the inlet enthalpy h1 is, h1 = 1474 BTU/lb If the condenser vacuum exists at a pressure of 0.6 PSI, a realistic value for a power plant condenser, then referring to the steam tables in the Van Wylen and Sonntag thermodynamics book, we find that the enthalpy h2 will be, h2 = 847 BTU/lb and the amount of useful work that the turbine can perform with the condenser in place would therefore be, W = h1 – h2 = 1474 BTU/lb – 847 BTU/lb = 627 BTU/lb So essentially with the condenser present, the work of the turbine is increased by 168 BTU/lb (627 BTU/lb – 459 BTU/lb). To put this increase into terms we can relate to, consider this. Suppose there’s one million pounds of steam flowing through the turbine each hour. Knowing this, the turbine power increase, P, is calculated to be, P = (168 BTU/lb) ´ (1,000,000 lb/hr) = 168,000,000 BTU/hr Now according to Marks’ Standard Handbook for Mechanical Engineers, a popular general reference book in mechanical engineering circles, one BTU per hour is equivalent to 0.000393 horsepower, or HP. So converting turbine power, P, to horsepower, HP, we get, P = (168,000,000 BTU/hr) ´ (0.000393 HP/BTU/hr) = 66,025 HP A typical automobile has a 120 HP engine, so this equation tells us that the turbine horsepower output was increased a great deal simply by adding a condenser to the turbine exhaust. In fact, it was increased to the tune of the power behind approximately 550 cars! What all this means is that the stronger the vacuum within the condenser, the greater the difference between h1 and h2 will be. This results in increased turbine efficiency and work output, as evidenced by the greater numeric value for W. Put another way, the turbine’s increased efficiency is a direct result of the condenser’s vacuum forming action and its recapturing of the steam that would otherwise escape from the turbine’s exhaust into the atmosphere. This wraps up our series on the power plant water-to-steam cycle. Next time we’ll use the power of 3D animation to turn a static 2D image of a centrifugal clutch into a moving portrayal to see how it works. ________________________________________ |
Posts Tagged ‘BTU’
How Condensers Increase Efficiency Inside Power Plants
Wednesday, December 4th, 2013Enthalpy Values in the Absence of a Condenser
Tuesday, November 26th, 2013|
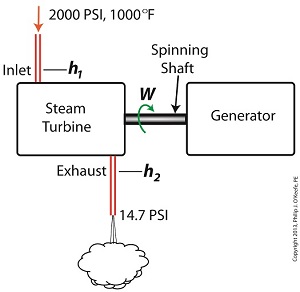
Last time we learned that the amount of useful work, W, that a steam turbine performs is calculated by taking the difference between the enthalpy of the steam entering and then leaving the turbine. And in an earlier blog we learned that a vacuum is created in the condenser when condensate is formed. This vacuum acts to lower the pressure of turbine exhaust, and in so doing also lowers the enthalpy of the exhaust steam. Putting these facts together we are able to generate data which demonstrates how the condenser increases the amount of work produced by the turbine. To better gauge the effects of a condenser, let’s look at the differences between its being present and not present. Let’s first take a look at how much work is produced by a steam turbine without a condenser. The steam entering the turbine inlet has a pressure of 2000 pounds per square inch (PSI) and a temperature of 1000°F. Knowing these turbine inlet conditions, we can go to the steam tables in any thermodynamics book to find the enthalpy, h1. Titles such as Fundamentals of Classical Thermodynamics by Gordon J. Van Wylen and Richard E. Sonntag list enthalpy values over a wide range of temperatures and pressures. For our example this volume tells us that, h1 = 1474 BTU/lb where BTU stands for British Thermal Units, a unit of measurement used to quantify the energy contained within steam or water, in our case the water to steam cycle inside a power plant. Technically speaking, a BTU is the amount of heat energy required to raise the temperature of one pound of water by one degree Fahrenheit. The term lb should be a familiar one, it’s the standard abbreviation used for pound, so enthalpy is the measurement of the amount of energy per pound of steam flowing through, in this case, the turbine. Since there is no condenser attached to the steam turbine’s exhaust in our illustration, the turbine discharges its spent steam into the surrounding atmosphere. The atmosphere in our scenario exists at 14.7 PSI because our power plant happens to be at sea level. Knowing these facts, the steam tables inform us that the value of the exhausted steam’s enthalpy, h2, is: h2 = 1015 BTU/lb Combining the two equations we are able to calculate the useful work the turbine is able to perform as: W = h1 – h2 = 1474 BTU/lb – 1015 BTU/lb = 459 BTU/lb This equation tells us that for every pound of steam flowing through it, the turbine converts 459 BTUs of the steam’s heat energy into mechanical energy to run the electrical generator. Next week we’ll connect a condenser to the steam turbine to see how its efficiency can be improved.
________________________________________ |
Coal Power Plant Efficiency
Sunday, July 11th, 2010|
Is there any price a man dying of thirst in the desert would not pay for a tall glass of cold water? What is the point at which Americans will decide they can do without heat, refrigerators, electric lights? My neighbor refuses to run the air conditioner, even when it’s 90 degrees and 90 percent humidity. They have obviously made the choice to sweat and be uncomfortable in their homes rather than pay high utility bills. Most of us are concerned with the environment, but when times are hard like they are now many of us become more concerned with our pocketbooks. Just as we need to make our financial ends meet, so do energy suppliers. Without a certain level of profit, their service to us will decline, and regular, dependable delivery of their precious commodities to us will suffer. If they were to go out of business, what then? Reading by candlelight may be romantic for a night or two, but nights on end? Let’s consider the energy provided by coal-fired power plants, for example. They’re in the electric utility business, and they provide us with the lion’s share of our energy. To keep a handle on operating costs, power plant engineers monitor how many British Thermal Units (BTUs) of heat energy are going into the power generation process versus how many kilowatt-hours of electricity are coming out. What’s a BTU and what does it matter to us? Well, it’s the amount of heat energy your kitchen stove uses to raise the temperature of one pint of water by one degree Fahrenheit. As for a kilowatt-hour, that’s a thousand watts of power produced over the space of an hour– enough to light ten 100 watt light bulbs. Now that we’ve explained the key term, we can explore the notion of heat rate, terminology very important to efficient power plant operation. Heat rate is simply the ratio of BTUs to kilowatt-hours. So what’s the importance of monitoring heat rate? For one thing, in order to get the most bang for your buck you want to keep the heat rate as low as possible. When the heat rate is high, you’re burning more coal than you have to because you’re wasting heat energy. This results in higher electricity costs to the consumer. This is exactly the situation at play when low sulfur coals are used as compared to the better burning coals of yester-year. So where does the wasted heat energy go if it isn’t being converted into electrical energy? For one thing, it can be lost through steam and water leaks in the power plant piping system. There are other losses too. Another way to lose heat energy is when thermal insulation is missing from pipes, causing heat to escape into the atmosphere. The opposite side of inefficiency is presented by the problem of too much heat energy building up, unable to be transferred to the steam. This is the result if ash is allowed to accumulate inside the boiler, acting as a thermal insulator. The heat has nowhere to go except up the smoke stack and into the atmosphere. Needless to say it’s important to keep heat rate as low as possible by keeping power plant equipment insulated and in good repair. But there are some things that affect heat rate that we just can’t do anything about, they’re known as “uncontrollable factors,” and we’ll learn about them next week. _____________________________________________ |