|
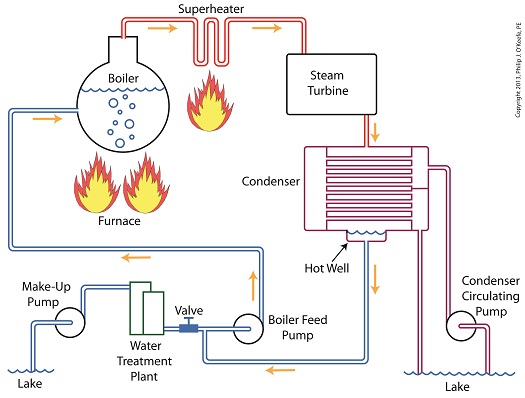
Last time we learned how the condenser within a power plant acts as a conservationist by transforming steam from the turbine exhaust back into water. This previously purified water, or condensate, contains valuable residual heat energy from its earlier journey through the power plant, making it perfect for reuse within the boiler, resulting in both water and fuel savings for the plant. Today we’ll take a look at a highly pressurized form of condensate known as boiler feed water and how it helps the power plant save money by recycling residual heat energy in the steam and water cycle. Let’s begin by integrating the condenser into the big picture, the complete water-to-steam power plant cycle, to see how it fits in. The illustration shows that both the make-up pump and the condenser circulating water pump draw water from the same supply source, in this case a lake. The circulating water pump continuously draws in water to keep the condenser tubes cool, while the make-up pump draws in water only when necessary, such as when initially filling the boiler or to make up for leaks during operation, leaks which typically occur due to worn operating parts. In a nutshell, the condenser recycles steam from the turbine exhaust for its reuse within the power plant. The journey begins when condensate drains from the hot well located at the bottom of the condenser, then gets siphoned into the boiler feed pump. If you recall from a previous article, the boiler feed pump is a powerful pump that delivers water to the boiler at high pressures, typically more than 1,500 pounds per square inch in modern power plants. After its pressure has been raised by the pump, the condensate is known as boiler feed water. The boiler feed water leaves the boiler feed pump and enters the boiler, where it will once again be transformed into steam, and the water-to-steam cycle starts all over again. That is, boiler feed water is turned to steam, it’s superheated to drive the turbine, then condenses back into condensate, and finally it’s returned to the boiler again by the boiler feed pump. Trace its journey along this closed loop by following the yellow arrows in the illustration. While you were following the arrows you may have noticed a new valve in the illustration. It’s on the pipe leading from the water treatment plant to the boiler feed pump. Next time we’ll see how this small but important item comes into play in the operation of our basic power plant steam and water cycle. ________________________________________ |
Posts Tagged ‘power plant engineer’
Boiler Feed Water, A Special Kind of Condensate
Tuesday, October 22nd, 2013How A Power Plant Condenser Works, Part 3
Monday, October 14th, 2013|
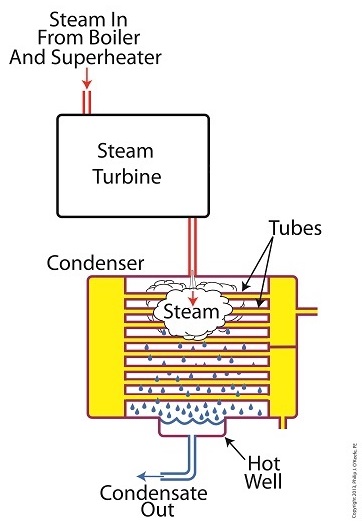
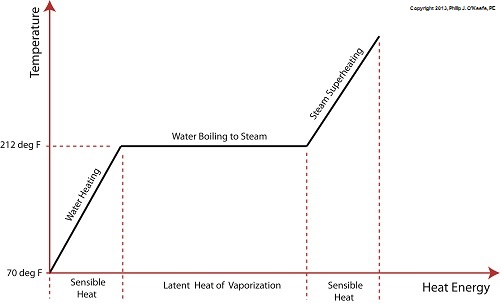
We’ve been discussing various aspects of a power plant’s water-to-steam cycle, from machinery specifics to identifying inefficiencies, and today we’ll do more of the same by introducing the condenser hot well and discussing its importance as a key contributor to the conservation of energy, specifically heat energy. Let’s start by returning our attention to the steam inside the condenser vessel. Last week we traced the path of the condenser’s tubes and learned that the cool water contained within them serve to regulate the steam’s temperature surrounding them so that temperatures don’t rise dangerously high. To fully understand the important result of this dynamic we have to revisit the concept of latent heat energy explored in a previous article. More specifically, how this energy factors into the transformation of water into steam and vice versa. Steam entering the condenser from the steam turbine contains latent heat energy that was added earlier in the water/steam cycle by the boiler. This steam enters the condenser just above the boiling point of water, and it will give up all of its latent heat energy due to its attraction to the cool water inside the condenser tubes. This initiates the process of condensation, and water droplets form on the exterior surfaces of the tubes. The water droplets fall like rain from the tube surfaces into the hot well situated at the bottom of the condenser. This hot well is essentially a large basin that serves as a collection point for the condensed water, otherwise known as condensate. It’s important to collect the condensate in the hot well and not just empty it back into the lake, because condensate is water that has already undergone the process of purification. It’s been made to pass through a water treatment plant prior to being put to use in the boiler, and that purified water took both time and energy to create. The purified condensate also contains a lot of sensible heat energy which was added by the boiler to raise the water temperature to boiling point, as we learned in another previous article. This heat energy was produced by the burning of expensive fuels, such as coal, oil, or natural gas. So it’s clear that the condensate collecting in the hot well has already had a lot of energy put into it, energy we don’t want to lose, and that’s why its an integral part of the water-to-steam setup. It acts as a reservoir, and the drain in its bottom allows the condensate to flow from the condenser, then follow a path to the boiler, where it will be recycled and put to renewed use within the power plant. Next week we’ll follow that path to see how the condensate’s residual heat energy is put to good use. ________________________________________ |
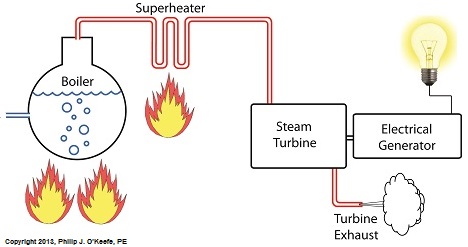
Superheater Construction and Function
Sunday, September 15th, 2013|
Power plants produce electrical energy for consumers to use, whether at home or for business, that’s obvious enough, but did you know that in order to produce that electrical energy they must first be supplied with heat energy? The heat energy that power plants crave comes from a fuel source, such as coal, oil, or natural gas, by way of a burning process. Once the heat energy is released from the coal through burning, it’s transported into a steam turbine by way of superheated steam, which is supplied to it by a piece of equipment named, appropriately enough, a superheater. So what is a superheater and how does it function? Take a look at the illustration below. The superheater looks like a W. It’s actually a cascading array of bent steam pipe, situated above a source of open flames which are produced by the burning of a fuel source. A photo of an actual superheater is shown below. So how many bends are in a superheater? Enough to fill the needs of the particular power plant it is supplying energy to. Since all power plants are designed differently, we’ll keep things in general terms. The many bends in the superheater’s pipes form a circuitous path for steam to flow as it follows a path from the boiler to the steam turbine. The superheater’s unique construction gives the steam flowing through it maximum exposure to heat. In other words, the bends increase the time it takes for the steam to flow through the superheater. The more bends that are present, the longer the steam will be exposed to the flame’s heat energy, and the longer that exposure, the more heat energy that is absorbed by the steam. Superheating routinely results in temperatures in excess of 1000°F. This superheated steam is laden with abundant heat energy which will keep the steam turbine spinning and the generator operating. The net result is millions of watts of electrical power. As we learned in a previous blog, the superheater is designed to provide the turbine with sensible heat energy to prevent steam from completely desuperheating, which would result in dangerous condensation inside the turbine. The newly added superheater is a major improvement to a power plant’s water-to-steam cycle, but there’s still plenty of waste and inefficiency in the system, which we’ll discuss next week.
________________________________________ |
Superheating, Part I
Monday, August 19th, 2013|
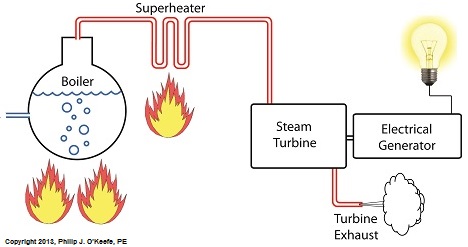
Last time we learned that our power plant boiler as presently designed doesn’t do a good job of producing ample amounts of superheated steam, the kind of steam that turbines need to spin and power generators. During the process of superheating the more heat energy that’s added to the steam in our boiler, the higher its temperature becomes. However, our boiler can only produce a limited amount of superheated steam as it stands now. So how do we get more heat energy into the superheated steam? Take a look at the illustration below for the solution to the problem. You’ll note a prominent new addition to our illustration. It’s called a superheater. The superheater performs the function of raising the temperature of the steam produced in our boiler to the incredibly high temperatures required to run steam turbines and electrical generators down the line, as explained in my blog on steam turbines. The superheater adds more heat energy to the steam than the boiler can alone. In fact, the amount of heat energy in the superheated steam produced with our new design is proportional to the amount of electrical energy that power plant generators produce. Its addition to our setup will result in more energy supplied to the turbine, which in turn spins the generator. The result is more electricity for consumers to use and a more efficiently operating power plant. But inefficiency isn’t the only problem addressed by the superheater. We’ll see what else it can do next week. ________________________________________ |
Heat Energy Within the Power Plant—The Power Behind the Turbines
Monday, July 29th, 2013|
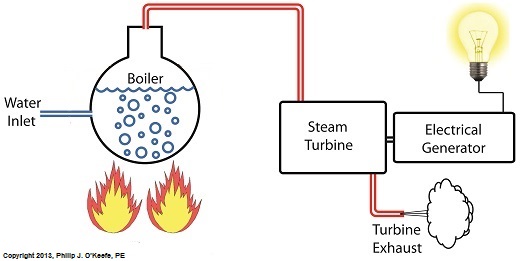
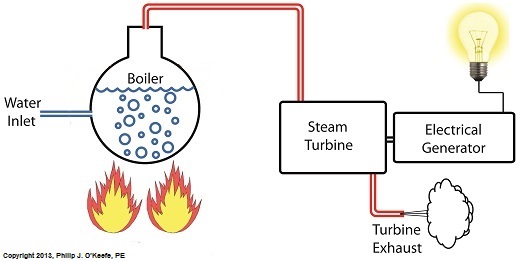
Last time we discovered that the boiling point of water varies. It’s dependent upon the amount of pressure exerted on its surface, which varies due to a variety of reasons, including where it is in relation to sea level. Before we see what happens under higher than atmospheric pressures, such as exist in an electric utility power plant boiler, let’s cover some basics. In the power plant, water is heated in a boiler specifically to produce steam, unlike our tea kettle where the primary purpose is to produce hot water. The steam produced is used to spin turbine generators, which in turn generate electricity, as I explained in a previous blog on steam turbines. Unlike a tea kettle, which is open to the atmosphere on your kitchen stove, the boiler in a power plant is an enclosed, reinforced steel vessel. See illustration below. The reinforced steel boiler vessel is designed to withstand great internal pressure as temperatures rise within. In addition to providing a safety feature, the enclosed space provides a sheltered environment for collecting steam so it can later be put to use spinning power generating turbines down the line. In other words, surface water inside the boiler is closed off from the surrounding atmosphere, allowing its internal pressure to build for our specific purposes. As heat energy is added to water within the boiler, the water boils and steam bubbles break out from its surface, filling the empty space above the surface with pressurized steam. This steam will try to expand here, but it can’t, because it’s being constrained by the reinforced steel vessel within which it is enclosed. Instead, steam pressure builds up on the surface of the water inside the boiler until it is high enough to be released through an attached pipe which is connected to a nearby turbine. We’ll talk more about this pent-up energy and how it is put to use within the power plant in next week’s blog. ___________________________________________
|
Coal Power Plant Fundamentals
Sunday, January 23rd, 2011| Several years ago I was asked by power producers within the electric utility industry to write and then present a training course on the subject of coal power plant fundamentals. The finished product was a two day introductory course on the energy transformation process within a coal fired plant.
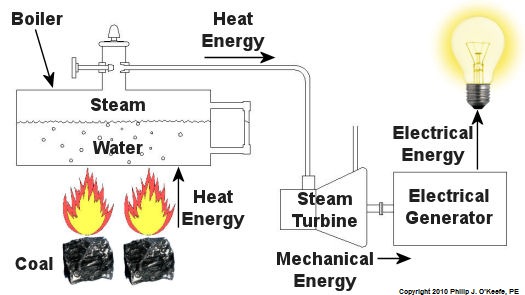
Since that time my seminar, entitled Coal Power Plant Fundamentals, has been presented to a variety of audiences, including Mirant Corporation, Platte River Power Authority, and Integrys Energy Group, Inc. Audience makeup has been diverse and has included equipment manufacturers, mining companies, power industry consultants, and regulatory agencies. This seminar, which I continue to present today in meeting rooms across the country, covers all major systems in a typical power plant, from coal handling when the coal first enters the plant, to its eventual end destination, the electrical switch yard which facilitates power transmission to customers. My Power Point presentation is embellished with ample illustrations, including photographs that I have taken during the course of my career and diagrams which I created using CAD, or Computer Aided Drawing software, one of which is featured below. In addition to the overhead slides, I provide a 150-page bound book which is distributed to seminar attendees. They use it to both follow along with my lecture and have a source of refresher material to take home with them. I’ve been told that having my illustrations in front of them makes a world of difference towards their understanding of the subject matter. The unique thing about my course is that it focuses on the simplified presentation of complex engineering concepts, much like my blogs do. Of course it always helps to have an engineering background or scientific background of sorts, but I wrote the course to accommodate understanding of the subject matter by individuals without any technical background. Accountants, salespersons, administrative staff, plant operating and maintenance workers, and journalists have all found the course to be easy to follow, interesting, and informative. So how do you get electricity from coal? To answer this question and give you a sampling of my seminar material let’s take a look at Figure 1. Figure 1 – The Coal Power Plant Energy Transformation Process Following along from left to right, the coal is first burned in order to transform the chemical energy which it contains into heat energy. That heat energy is then absorbed by water inside a nearby boiler, where it is converted into steam. The heat energy in the steam flows through a pipe into a steam turbine where it is again transformed, this time into mechanical energy that enables the turbine shaft to spin. The mechanical energy in the turbine is then transmitted by its shaft, enabling it to turn an electrical generator. And, finally, the mechanical energy is transformed by the generator into electrical energy for our usage. Simple process, right? Well, maybe, maybe not. My illustration certainly helped to simplify things, but there are a lot of details that were purposely omitted so as not to “muddy the waters.” It’s those details which have the potential to make things a lot more complicated, and next week we’ll begin to take a closer look at some of them. _____________________________________________ |
Coal Power Plant Efficiency
Sunday, July 11th, 2010|
Is there any price a man dying of thirst in the desert would not pay for a tall glass of cold water? What is the point at which Americans will decide they can do without heat, refrigerators, electric lights? My neighbor refuses to run the air conditioner, even when it’s 90 degrees and 90 percent humidity. They have obviously made the choice to sweat and be uncomfortable in their homes rather than pay high utility bills. Most of us are concerned with the environment, but when times are hard like they are now many of us become more concerned with our pocketbooks. Just as we need to make our financial ends meet, so do energy suppliers. Without a certain level of profit, their service to us will decline, and regular, dependable delivery of their precious commodities to us will suffer. If they were to go out of business, what then? Reading by candlelight may be romantic for a night or two, but nights on end? Let’s consider the energy provided by coal-fired power plants, for example. They’re in the electric utility business, and they provide us with the lion’s share of our energy. To keep a handle on operating costs, power plant engineers monitor how many British Thermal Units (BTUs) of heat energy are going into the power generation process versus how many kilowatt-hours of electricity are coming out. What’s a BTU and what does it matter to us? Well, it’s the amount of heat energy your kitchen stove uses to raise the temperature of one pint of water by one degree Fahrenheit. As for a kilowatt-hour, that’s a thousand watts of power produced over the space of an hour– enough to light ten 100 watt light bulbs. Now that we’ve explained the key term, we can explore the notion of heat rate, terminology very important to efficient power plant operation. Heat rate is simply the ratio of BTUs to kilowatt-hours. So what’s the importance of monitoring heat rate? For one thing, in order to get the most bang for your buck you want to keep the heat rate as low as possible. When the heat rate is high, you’re burning more coal than you have to because you’re wasting heat energy. This results in higher electricity costs to the consumer. This is exactly the situation at play when low sulfur coals are used as compared to the better burning coals of yester-year. So where does the wasted heat energy go if it isn’t being converted into electrical energy? For one thing, it can be lost through steam and water leaks in the power plant piping system. There are other losses too. Another way to lose heat energy is when thermal insulation is missing from pipes, causing heat to escape into the atmosphere. The opposite side of inefficiency is presented by the problem of too much heat energy building up, unable to be transferred to the steam. This is the result if ash is allowed to accumulate inside the boiler, acting as a thermal insulator. The heat has nowhere to go except up the smoke stack and into the atmosphere. Needless to say it’s important to keep heat rate as low as possible by keeping power plant equipment insulated and in good repair. But there are some things that affect heat rate that we just can’t do anything about, they’re known as “uncontrollable factors,” and we’ll learn about them next week. _____________________________________________ |