Posts Tagged ‘temperature’
Monday, April 16th, 2018
|
Ever hear the old saying, “There’s more than one way to cook a goose”? The statement is meant to encourage creative thinking when problem solving. This forward thinking can be applied to the problem of destructive cavitation bubbles as well. Finding ways to reduce cavitation is something engineers are well versed in. As discussed in our last blog, one way to prevent cavitation is by lowering water temperature at a centrifugal pump’s inlet. But sometimes that isn’t possible. Today we’ll discuss another way, reducing cavitation by increasing water pressure.
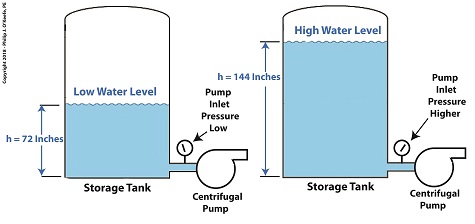
One way to Reduce Cavitation by Increasing Water Pressure
If you’ve ever seen a movie featuring divers, you’ll no doubt be aware that the deeper a diver goes, the more water pressure there is bearing down on him from above. The same goes for a centrifugal pump’s storage tank. The higher the water level inside the tank, the higher the pressure bearing down on the pump’s inlet, which is located at the bottom of the tank. This is the area in which cavitation bubbles are likely to form. The mathematical equation that illustrates this relationship is,
P = γ × h (1)
where, P is water pressure at the bottom of the tank, γ is the Greek symbol gamma, representing the specific weight of water, (0.036 pounds/inch3), and h is the depth of the water inside the tank.
Let’s see what happens when we increase the water level, h, from 72 inches, shown on the left, to 144 inches, on the right.
P = (0.036 Lb/in3) × (72 in) = 2.592 PSI (2)
When the water level is raised to 144 inches, P becomes,
P = (0.036 Lb/in3) × (144 in) = 5.184 PSI (3)
We see that by raising the water level in the tank from 72 to 144 inches, pressure at the bottom of the tank where the inlet is located is increased from 2.592 PSI to 5.184 PSI, pounds per square inch.
Next time we’ll see how simply elevating the tank has an impact on cavitation.
Copyright 2018 – Philip J. O’Keefe, PE
Engineering Expert Witness Blog
____________________________________ |
Tags: cavitation, cavitation bubbles, centrifugal pump, engineering, pressure, pump inlet, specific weight of water, storage tank, temperature, water level
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, power plant training, Product Liability | Comments Off on One way to Reduce Cavitation by Increasing Water Pressure
Monday, April 9th, 2018
|
As we learned previously, cavitation bubbles form at a centrifugal pump’s inlet when the thermodynamic properties of water, namely temperature and pressure, are right. Today we’ll see how just manipulating water temperature can control cavitation.

Manipulating Water Temperature to Control Cavitation
Some centrifugal pumps draw water from an external heat source such as a heat exchanger in order to provide heat to buildings, generate power, and perform manufacturing processes. On some exchangers heat is applied at a fixed rate and can’t be varied. On others heat can be varied by using a heat exchanger fitted with a temperature control. This makes it easy to reduce or lower water temperature introduced at the pump’s inlet. If the temperature is kept low enough relative to the pressure at the inlet, cavitation bubbles won’t form.
Let’s say water enters the pump’s inlet from a heat exchanger at 59ºF and internal pump pressure is 0.25 pounds per square inch (PSI). With these parameters in place water boils and cavitation bubbles will form in the pump inlet. But if the heat exchanger is adjusted so that temperature is lowered by a mere two degrees to 57ºF, cavitation ceases. This is in accordance with the boiling points of water, listed for various pressures and temperatures, as published in engineering thermodynamic texts.
If it’s not possible to lower water temperature at the pump inlet, an alternate method to control cavitation is to raise water pressure, which can be accomplished in different ways. We’ll review those options next time.
Copyright 2018 – Philip J. O’Keefe, PE
Engineering Expert Witness Blog
____________________________________ |
Tags: cavitation, centrifugal pump, engineering, heat exchanger, pressure, temperature, temperature control, thermodynamics
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, power plant training, Product Liability | Comments Off on Manipulating Water Temperature to Control Cavitation
Sunday, January 28th, 2018
|
Last time we learned how the thermodynamic properties of water contribute to the phenomenon of cavitation, and how liquids exist in three states, solid, liquid, or vapor, depending on temperature and surrounding air pressure. Our example of an open pot of water being heated on a stove demonstrated that once water temperature rose above 212ºF, it changed to steam, which initiated the cavitation process. Today we’ll see how decreasing pressure contributes to cavitation.
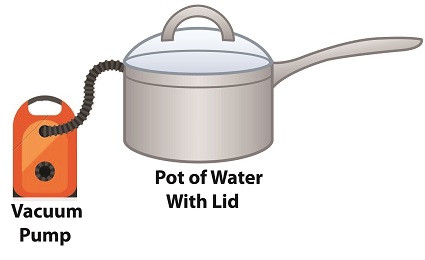
How Decreasing Pressure Contributes to Cavitation
Cavitation can occur without a heat source. In our pot example, we can start the cavitation process by simply decreasing the pressure of the air resting on top of the water, thereby also decreasing the water’s pressure.
Normally atmospheric pressure on Earth exists at around 15 pounds per square inch (PSI). But if we introduce a vacuum pump to an enclosed space, we can create an internal pressure which is lower than the surrounding atmospheric pressure outside the pot. In other words, we create a vacuum. A vacuum is any air pressure lower than atmospheric pressure. This vacuum environment produces an entirely new set of circumstances under which cavitation can occur. In fact, creating a vacuum makes it possible to boil water without using any heat!
As we learned in a past blog on the different forms of heat energy, the boiling point of water varies depending on the location of the stovetop, whether it’s in a place of low altitude, like New Orleans, or higher altitude, like Denver. But if we apply a tight lid to the pot and isolate its internal atmosphere from surrounding atmospheric pressure, you create a closed environment. This allows us to manipulate the pot’s internal pressure. When we attach a vacuum pump to remove air, we reduce the air pressure bearing down on the water inside. With much of the air removed, pressure inside the pot drops below normal atmospheric pressure existing outside the pot, and we discover that at 0.25 PSI water turns to steam at a mere 59ºF and cavitation can begin. That’s right, you can boil water without using heat.
Next time we’ll apply our knowledge of water pressure and temperature to an industrial setting and see how cavitation occurs inside pumps.
opyright 2018 – Philip J. O’Keefe, PE
Engineering Expert Witness Blog
____________________________________ |
Tags: atmospheric pressure, cavitation, temperature, thermodynamics, vacuum
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, power plant training, Product Liability | Comments Off on How Decreasing Pressure Contributes to Cavitation
Monday, September 2nd, 2013
|
Last time we learned that the addition of a superheater to the electric utility power plant steam cycle provides a ready supply of high temperature steam, laden with heat energy, to the turbine, which in turn powers the generator. But this isn’t its only job. One of the superheater’s most important functions is to regulate the ongoing process of desuperheating that takes place as the turbine consumes heat energy. To understand this, let’s see what takes place if the superheater were to be removed from its position between the boiler and turbine.
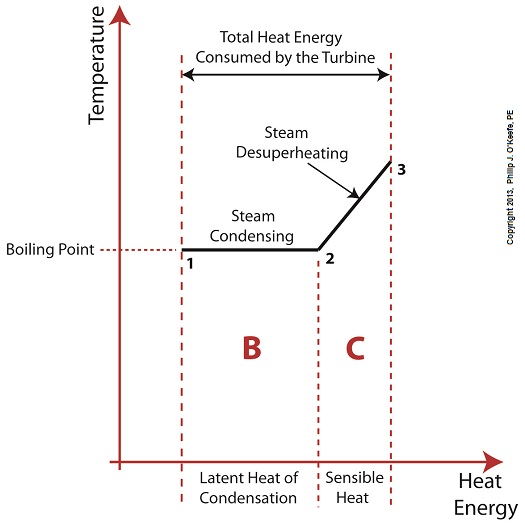
Figure 1
Without the superheater, the only available remaining source of sensible heat energy to the turbine would come from the meager amount present in phase C steam as shown in Figure 1. If you’ll recall from a past blog, the sensible heat energy contained in superheated steam is the best source of energy for a steam turbine, because it’s able to keep it operating most efficiently.
As the turbine consumes the heat energy in phase C, starting at point 3 and continuing to point 2, the steam it’s consuming is in the process of desuperheating, as evidenced by the downward slope between the two points. Desuperheating is an engineering term which means that as sensible heat energy is removed from the steam due to its use by the turbine, there will be a resulting drop in steam temperature. And if this process were to continue without the compensatory function provided by the addition of a superheater to the steam cycle, the steam’s temperature would eventually return to mere boiling point, at point 2. This is an undesirable thing.
With the steam’s temperature at boiling point, the only remaining source of heat energy to the turbine is the latent heat energy of phase B. This heat energy will lead to an undesirable circumstance for the operation of our power hungry turbine as we will see next week.
________________________________________ |
Tags: boiler, desuperheat, desuperheating, electric utility power plant, engineering expert witness, forensic engineer, generator, heat energy, latent heat energy, power, power plant engineering, power plant training, sensible heat energy, steam cycle, steam temperature, steam turbine, superheated steam, superheater, temperature
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, power plant training | Comments Off on Desuperheating in the Steam Turbine
Monday, July 15th, 2013
|
If you took high school chemistry, you learned that water is created when two gases, hydrogen and oxygen are combined. You may have even been lucky enough to have a teacher who was able to perform this magical transformation live during class.
Depending primarily on the amount of heat energy absorbed, water exists in one of the three states of matter, gas, liquid, or solid. Its states also depend on surrounding atmospheric pressure, but more about that later. For our discussion, the water will reside at the atmospheric pressure present at sea level, which is around 14.7 pounds per square inch.
Last time we learned that the heat energy absorbed by water before it begins to boil inside our example tea kettle is known as sensible heat within the field of thermodynamics. The more sensible heat that’s applied, the more the water temperature rises, but only up to a point.
The boiling point of water is 212°F. In fact this is the maximum temperature it will achieve, no matter how much heat energy is applied to it. That’s because once this temperature is reached water begins to change its state of matter so that it becomes steam. At this point the energy absorbed by the water is said to become the latent heat of vaporization, that is, the energy absorbed by the water becomes latent, or masked to the naked eye, because it is working behind the scenes to transform the water into steam.
As the water in a tea kettle is transformed into steam, it expands and escapes through the spout, producing that familiar shrill whistle. But what if we prevented the steam from dispersing into the environment and continued to add heat energy? Ironically enough, under these conditions temperature would continue to rise, upwards of 1500°F, if the stove’s burner were powerful enough. This process is known as superheating. Now hold your hats on, because even more ironically, the heat added to this superheated steam is also said to be sensible heat.
Confused? Let’s take a look at the graph below to clear things up.
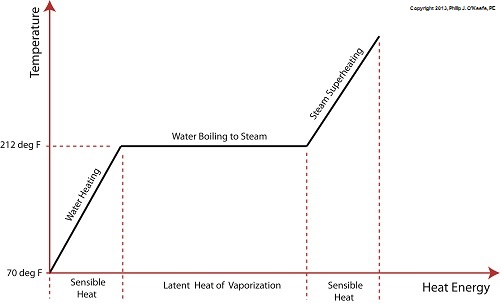
Sensible heat is heat energy that’s added to water, H2O, in its liquid state. It’s also the term used to describe the heat energy added to steam that’s held within a captive environment, such as takes place during superheating. On the other hand, the latent heat of vaporization, that is the heat energy that’s applied to water once it’s reached boiling point, does not lead to a further rise in temperature, as least as measured by a thermometer.
Next time we’ll see how surrounding air pressure affects water’s transition from liquid to steam.
___________________________________________
|
Tags: air pressure, atmospheric pressure, boil, boiler, boiling point, change of state, energy absorbed by water, engineer, engineering expert witness, expands, expert witness, forensic engineer, heat energy, heat transfer, latent heat, power engineering, power plant engineer, sensible heat, steam, steam expansion, superheat, superheating, temperature, temperature rise, thermodynamics, thermometer, water
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Innovation and Intellectual Property, Personal Injury, power plant training | Comments Off on Forms of Heat Energy – Latent
Sunday, July 7th, 2013
| In our house the whistle of a tea kettle is heard throughout the day, no matter the temp outside. So what produces that familiar high pitched sound?
When a tea kettle filled with room temperature water, say about 70°F, is heating on the stove top, the heat energy from the burner flame will transfer to the water in the kettle and its temperature will steadily rise. This heat energy that is absorbed by the water before it begins to boil is known as sensible heat in thermodynamics. To read more about thermodynamics, click on this hyperlink to one of my previous blog articles on the topic.
So, why is it called sensible heat? It’s so named because it seems to make sense. The term was first used in the early 19th Century by some of the first engineers who were working on the development of boilers and steam engines to power factories and railways. Simply stated, it’s sensible to assume that the more heat you add to the water in the kettle, the more its temperature will rise.
So how high will the temperature rise? Is there a point when it will cease to rise? Good questions. We’ll answer them next week, along with a discussion on another form of heat energy known as the latent heat of vaporization.
___________________________________________
|
Tags: boiler, engineering expert witness, forensic engineer, heat energy, latent heat of vaporization, power plant engineering, sensible heat, steam, temperature, thermodynamics, water
Posted in Engineering and Science, Expert Witness, Forensic Engineering, power plant training | Comments Off on Forms of Heat Energy – Sensible
Sunday, October 30th, 2011
| How do parents make life safer and healthier for their kids? One of the ways is to impose limits on things like roaming distance within the neighborhood, curfews, and insisting that you eat your vegetables. Just common sense, right? Let’s take a look at some more of it.
Limits are also necessary within the food manufacturing industry. Let’s take a look at Hazard Analysis and Critical Control Point (HACCP) Principle No. 3 to see how they’re established and why.
Principle 3: Establish critical limits for each critical control point. – You can think of a critical limit as a boundary of safety for each critical control point (CCP). So how do you determine that boundary of safety? It’s difficult to generalize, but if you’ve ever watched the TV show Hoarders, you have an excellent example of one that has not only been breeched, but torn asunder.
In order to prevent things in the commercial food industry from getting anywhere near Hoarders bad, maximum and minimum values are set in place, representing safeguards to physical, biological, and chemical parameters at play within the industry. Critical limits can be obtained from regulatory standards and guidelines, scientific literature, experimental studies, as well as information provided by consultants. These critical limits come into play with issues as varied as machine design, raw material temperatures, and overall safe processing times.
How could the hoarders let things get so bad? If you listen carefully, you’ll hear bits of information that provide a clue. They’ll say it started with a few things falling to the floor which they didn’t feel like picking up and it escalated from there.
Now all of us live within environments which differ as to their cleanliness, but by and large we live within space where we feel comfortable and consider to be reasonably clean. We don’t all habitually move stoves and refrigerators to clean, for example. But if we were so inclined, refrigerators do come with front access panels that are easily removed. Trouble is the space they provide access to often isn’t large enough to accommodate hands and a vacuum cleaner nozzle comfortably. You can imagine how frustrating and potentially dangerous it would be to public health to have commercial machinery that provided such limited access for cleaning.
To cope with this problem design engineers institute minimum and maximum parameters, such as in the critical limit dimensions of a removable cover. Their guideline would ensure that enough space is provided so that personnel can fully access all aspects of machinery with tools for cleaning. That same cover can also have established maximum critical limits, so that dimensions aren’t too large and heavy to be manipulated by hand. Human nature being what it is, something that is too difficult to remove may be “forgotten” and parts of the machine may never get cleaned.
Raw meats and many produce can contain hazards like salmonella, E. coli, and other nasty critters that are dangerous to human health. One of the ways the commercial food industry works to ensure that these contaminants aren’t unleashed on the public is to install programmable control systems into processing machinery that essentially cooks the meat at an established minimum temperature for a minimum amount of time. Utilizing this type of temperature control in conjunction with an established maximum cooking parameter for temperature and time will virtually eliminate the possibility of overcooked or burnt food products. When you buy that frozen dinner in most cases it’s completely cooked, but it’s a rarity to find it’s been burned.
Another situation in which critical limits are utilized is in the maintenance of machinery, such as when they limit the number of hours a machine can be operated before it is shut down for servicing.
Next week we’ll move on to Principle No. 4 and see how it establishes monitoring requirements for each CCP. ____________________________________________
|
Tags: access cover, bacteria, burnt food, CCP, cleaning, cooking, cooking parameter, critical limits, E. coli, engineering expert witness, experimental studies, FDA, food equipment design, food manufacturing industry, food processing machinery, food procution line, food safety, forensic engineer, HACCP, HACCP Principle 3, Hazaard Analysis and Critical Control Point, industrial control system, programmable control system, programmable logic controller, raw meat, regulatory standards, salmonella, temperature, temperature control, time
Posted in Engineering and Science, Expert Witness, Forensic Engineering, Personal Injury, Product Liability, Professional Malpractice | Comments Off on Food Manufacturing Challenges – HACCP Design Principle No. 3










